Computational Molecular Biology 2016, Vol.6, No.1, 1-20
10
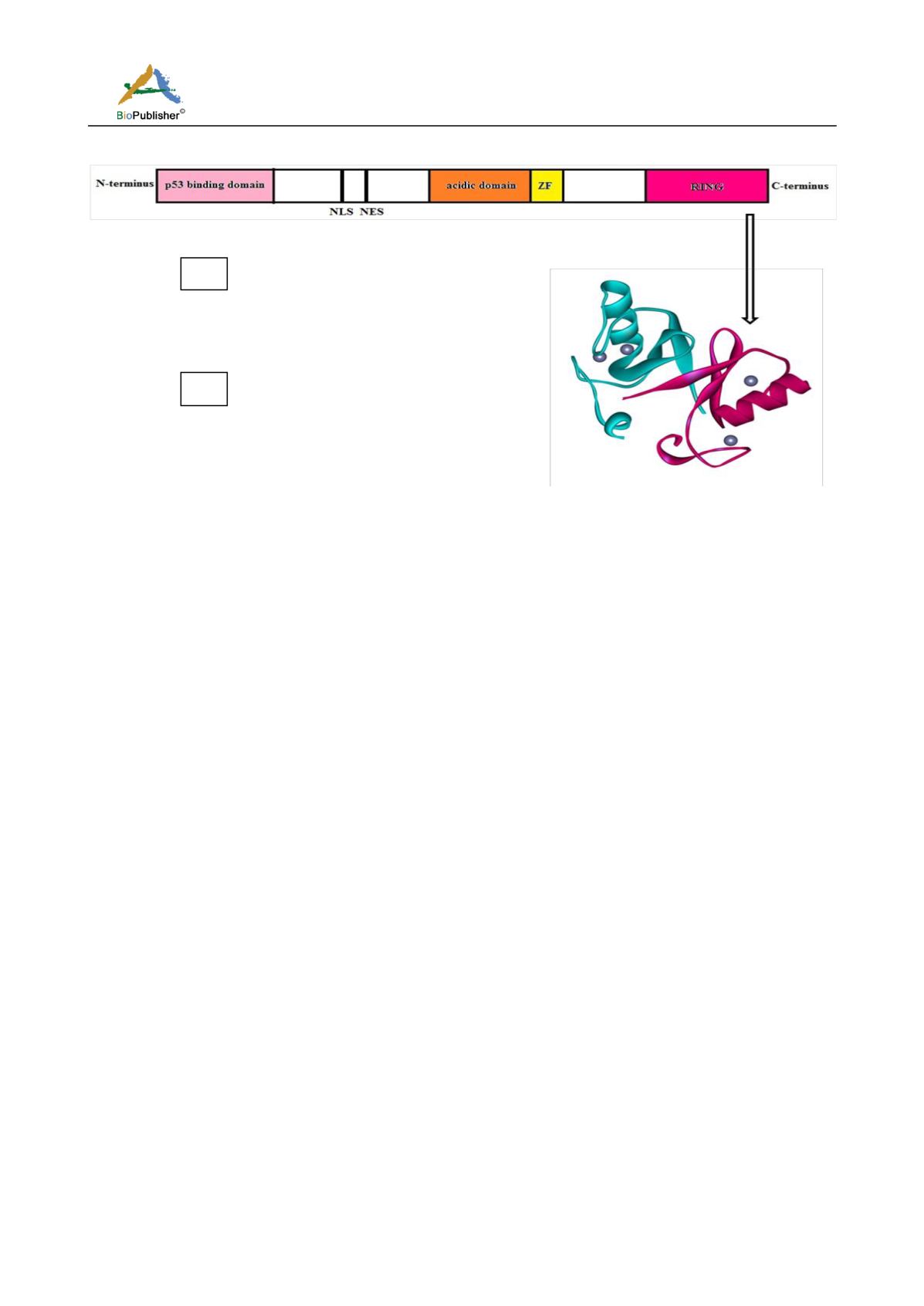
Figure 5 MDM2 E3 ligase
Note: (a) domain architecture shows N-terminal p53 binding domain, preceded by an acidic domain (orange) and a zinc finger motif
(yellow) and a C-terminal RING domain (magenta). Nuclear localisation signal (NLS) and nuclear export signal (NES) are present in
between p-53 binding domain and acidic domain. The RING domain consists of an nucleolar localisation signal (NoLS). (b) Ribbon
representation shows binding of MDM2 (magenta) binding to MDMX (blue) by their respect ive RING domains (PDB ID: 2VJE).
Grey balls represents zinc ion.
As MDM2 negatively regulates p53 tumor suppressor protein in the cell, molecules inhibiting the activity of MDM2 can be
beneficial in cancer therapy. Also, as a functional MDM2 is either a homodimer or a heterodimer with MDMX interacting with their
RING domains, inhibitors of the RING domain may be attractive in drug therapy for targetingMDM2.
Mdmx consists of a RING domain but lacks ubiquitin activity. Mdm2 interacts with Mdmx via their respective
RING domains(Tanimura et al., 1999). MDM2 is also known to ubiquitinate the DNA binding domain (DBD) of
p53 in addition to C-terminal domain (Chan et al., 2006). By attaching ubiquitin in Lys residues in the DBD
domain of p53, MDM2 mediates nuclear export of p53(Gu et al., 2001). MDM2 regulates its cellular
concentration by undergoing autoubiquitination. Self-ubiquitination increases its substrate’s ubiquitin ligase
activity, as ubiquitinated MDM2 recruits more E2 and increases its concentration (Ranaweera and Yang, 2013).
The discovery that inhibition of proteosome can induce apoptosis in cancer cells has made the UPS a centre of
attraction as a drug target in cancer progression. The FDA in 2003 approved the drug bortezomib which inhibits
the proteosome by binding to its β5 sub-unit reversibly. This results in the crowding of polyubiquinated proteins in
the cell (Chen et al., 2011). Although bortezomib proved to be effective in clinical trials, the effectiveness of the
drug is very narrow and also it confers cyto-toxic effects due to accumulation of misfolded proteins in the
endoplasmic reticulum. This activates the unfolded protein response (UPR) in the cell which ultimately results in
massive tumor necrotic response (Obeng et al., 2006). Carfilzomib was approved by FDA in 2012, it irrevesibly
inhibits the proteosome to a site other than bortezomib and is used for the treatment of lymphoma and multiple
myeloma (McBride et al., 2015) . Rather than blocking the general proteosome, inhibitors which target a
particular E3 ligase could be more pharmacologically specific with less cyto-toxic effect. Majority of the small
molecule inhibitors known to inhibit the E3 ligase are known to target the RING-type E3 ligases. Pyrrolidine
dithiocarbamate (PDTC) and its analogues have proved to be potent inhibitors of the NFkB pathway; they bind to
the RING domain of the E3 ligases to affect its function. Studies show pyrrolidine ring binds to the Cys residues
at the zinc binding site and breaks the bonds. As a result, the zinc ion pops out of the structure and the whole
protein structure collapses (Carta et al., 2012; Cvek and Dvorak, 2007; Zhang et al., 2011). The major small
molecule inhibitors of different E3 ligases are listed in Table 1.
(a)
(b)


