Computational Molecular Biology 2016, Vol.6, No.1, 1-20
9
4.1.2.3 SCF
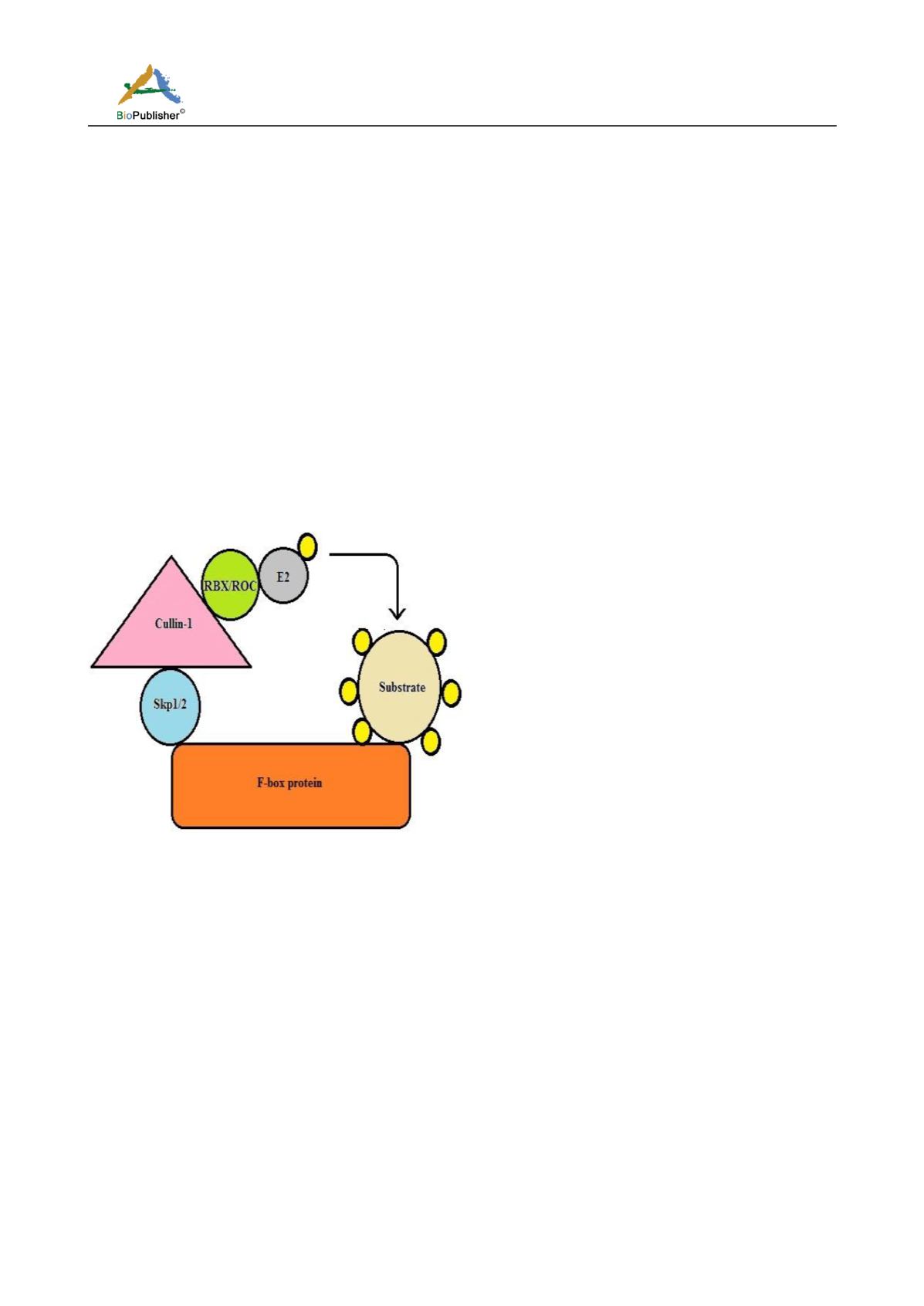
SCF (Skp1, cullins, F-box) are a class of multisubunit RING E3 ligases. It is the largest E3 ubiquitn ligase family
and is involved in regulation of ~20% protein regulated by the UPS. The SCF comprises of four structural
domains: a F-box protein which binds substrates and confers substrate specificity, SKP-1 which acts as an adaptor
protein, cullin (CUL -1,-2,-3,-4,-5 and -7) which acts as a scaffold protein and RBX/ROC RING also known as
SAGs (sensitive to apoptosis) proteins. The cullin protein binds SKP-1 and F-box protein in its N-terminus and
the RBX/ROC RING in its C-terminus (Figure 4). The CUL/RBX mediates the ligase activity by transferring the
ubiquitin from E2 to the substrate. Human genome is known to code around 69 F-box proteins, with only few of
them being well studied. The F-box protein binds the SKP-1 and cullin protein by the F-box domain and
substrates by the leucine rich or WD40 domains (Jia and Sun, 2012; Zheng et al., 2002). Cullin in the cytosol is
inhibited by CAND1, neddylation of cullin disrupts its association with CAND1 and makes it functional(Merlet et
al., 2009). Majority of SCF regulated substrates are involved in various cell signaling cascades. Evidences suggest
malfunctioning SCF in cancer progression(Skaar et al.,, 2014). Fbw7 an F-box protein , is a tumor suppressor
(Welcker and Clurman, 2008) and is found to be mutated in many cancers(Calhoun et al., 2003; Jardim et al.,
2014). Over expression of the SCF component Skp2, which acts as an oncogenic is related to cancer progression
(Gstaiger et al., 2001; G. Yang et al., 2002). The many components of SCF RING E3 ligases could be regulated at
various levels making them an attractive drug target.
Figure 4 Schematic diagram of SCF E3 ubiquitin ligase
Note: F-box (orange) provides the specificity by binding to the substrate. Skp1/2 (blue) acts as a linker between cullin-1 (pink) and
F-box proteins. RBX/ROC (green) is the RING proteins which bind to E2 enzyme to mediate the process of ubiquitination. It
transfers the ubiquitin molecule (yellow) from E2 to the substrate.
4.1.2.4 MDM2
Tumour suppressor protein p53 is regulated in two ways: first by post-translational regulation and second by the
RING E3 ligases, MDM2 and MDMX. Functional MDM2 is either a homodimer or a heterodimer with MDMX.
MDM2 works as an antagonist in regulation of p53. Murine double minute 2 (MDM2) known as HDM2 in
humans, binds and blocks the N-terminal transactivation domain (TAD) of p53 protein and targets p53 for
ubiquitin-proteosomal degradation(Wade et al., 2010). MDM2 comprises a p53-binding domain in its N-terminus
followed by an acidic domain and a zinc finger domain. The RING finger domain containing the nucleolar
localization signal (NoLS) lies at the C-terminal end. The structure of MDMX is similar to MDM2, the acidic
domain is shorter. MDM2 consists of nuclear localization signal (NLS) and nuclear export signal (NES) which
lies in between the p53 binding domain and the ac idic domain, these features are missing from the structure of
MDMX (Figure 5).


