Journal of Mosquito Research 2015, Vol.5, No.11, 1-6
5
3.2 Deltamethrin (KOTab 1-2-3
®
) and plant materials
acquisition and preparation
Deltamethrin (KOTab 1-2-3
®
)
was acquired and
prepared as described (Yugi et al., 2015). Fresh leaves
(shoot and midsection) and mature green fruits of
Endod and Fresh leaves of
Azadirachta indica
(Neem),
were acquired, identified and voucher specimen
deposited as described elsewhere (Yugi et al., 2015).
The plant parts were dried in a shade at room
temperature, grounded and extracts obtained using
ethanol and water as described in details elsewhere
(Tilahun et al., 2003; Parekh et al., 2005; Das et al.,
2010; Yugi et al., 2015).
The extracts were concentrated by freeze drying using
a rotary vacuum evaporator at 40-42°C to obtain
essential oil that was then kept in airtight glass bottles
to serve as stock quantity. From the freeze-dried stock,
80mg were weighed and serial dilutions made to obtain
different concentrations of 40, 20, 10, 10, 5 and 2.5 mg
in 100mls of rain harvested water.
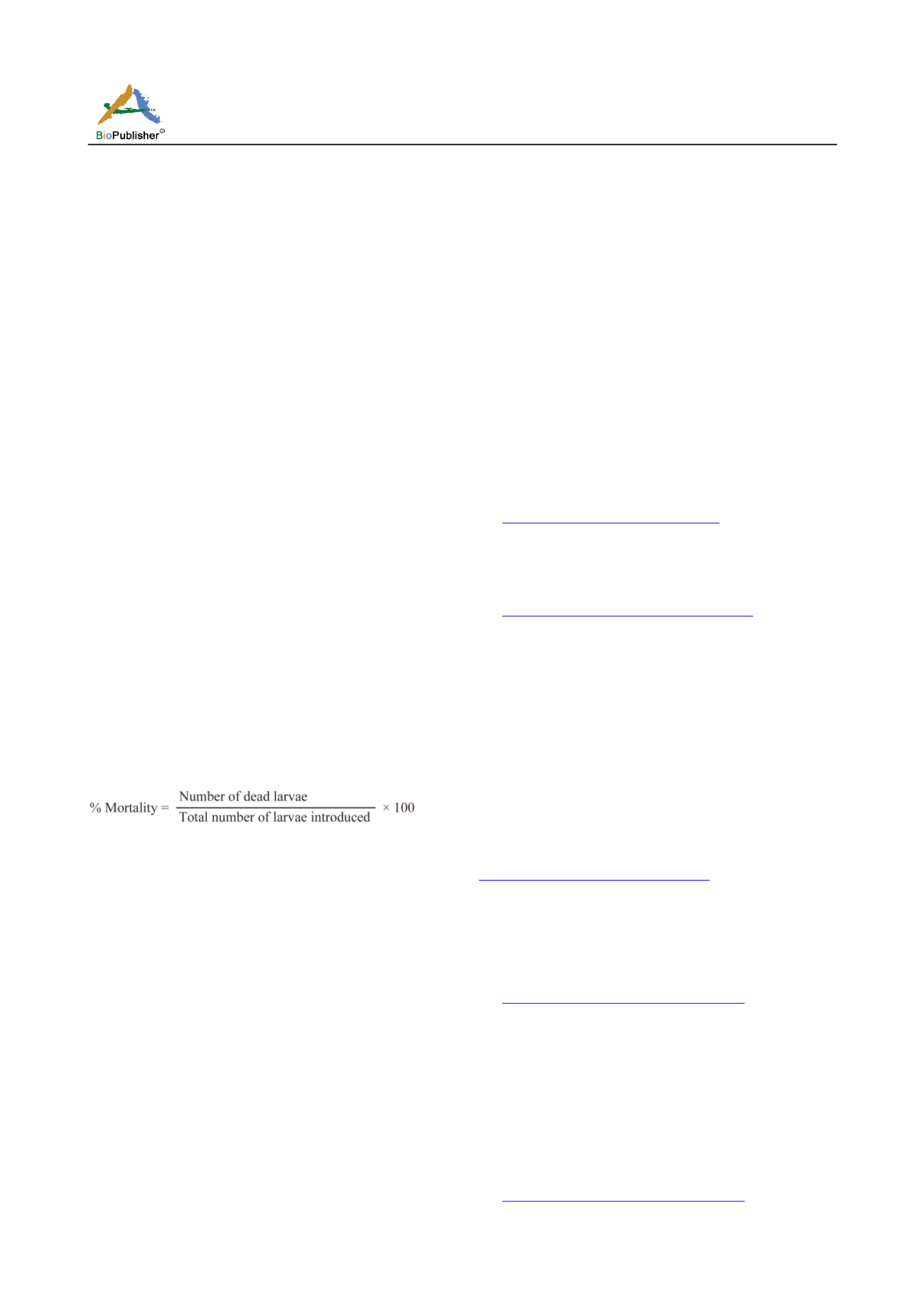
3.3 Larvicidal bioassays
Larvicidal activities were tested in accordance with
the WHO procedure (WHO, 1996) and as described
elsewhere (Yugi et al., 2015). Mortality rate were
registered after 24 hour exposure period and larval
mortality calculated for each concentration using the
formula;
Standard WHO procedures were used to assess
effectiveness of the extracts as larvicide at a mortality
rate of > 80% (WHO, 2005). Moribund and dead
larvae were collected and disposed off in a septic tank.
3.4 Data analysis
Data obtained from the bioassays was entered in excel
spreadsheets for ease of handling. The relationship
between the effective doses of the extracts with respect
to parts of Endod used was determined using
descriptive statistics. One way analysis of variance
(ANOVA) was used to assess the level of significance
of the various doses on larvae mortality. All statistical
analysis was performed using SAS statistical package
version 20.
Competing interest
The authors declare that they have no competing interest.
Acknowledgements
We thank Patience Akoth, Charlotte Awuor, Dalton Ochieng’
Trevor Omondi and Richard Amito, for helping culture all
mosquitoes used in this study. We thank Kisumu Polytechnic
for equipments for the extractions of the crude extracts from
Endod and Neem plants, Centre for Global Health Research/Kenya
Medical Research Institute (CGHR/KEMRI) for mosquitoes,
laboratory space and equipments, VIRED International for
providing transportation and logistics for sourcing for Endod
and Neem parts. We also thank National Commission for
Science, Technology and Innovation (NACOSTI), Kenya for
funding this project.
References
Anupam G., Nandita C., and Goutam C., 2012, Plant extracts as potential
mosquito larvicides. Indian Journal of Medical Research, 135: 581-598
Boutayeb, A., 2006, The Double burden of communicable and non-commu-
nicable diseases in developing countries.
Transactions of the Royal
Society of Tropical Medicine and Hygiene
, 100(3): 191-199
Cartilla P., and De la Cruz J., 2012, Termiticidal Potential of
Stachytarpheta
Jamaicensis (L.)
Vahl, 1: 1-5
Charles C., and Nielsen-LeRoux C., 2000, Mosquitocidal Bacterial Toxins:
Diversity, Mode of Action and Resistance. Phenomena, 95(Suppl 1):
201-206
Das K., Tiwari R.K.S., and Shrivastava D.K., 2010, Techniques for
evaluation of medicinal plant products as antimicrobial agent: Current
methods and future trends. Journal of Medicinal Plants Research, 4(2):
104-111
Das N.G., Goswami D., and Rabha B., 2007, Preliminary evaluation of
mosquito larvicidal efficacy of plant extracts. Journal of Vector Borne
Diseases, 44: 145-148
Joshi B., Sah G.P., Basnet B.B., Bhatt M.R., Sharma D., Subedi K., Pandey
J., and Malla R., 2011, Phytochemical extraction and antimicrobial
properties of different medicinal plants
: Ocimum sanctum
(Tulsi),
Eugenia
caryophyllata
(Clove),
Achyranthes bidentata
(Datiwan) and
Azadirachta
indica
(Neem). Journal of Microbiology andAntimicrobials, 3(1): 1-7
Jude D.B., Petola A.S., Sali A.N., Judith L.N., Maximilienne N., Julius E.O.,
and Rose G.F.L., 2013, Larvicidal and Repellent Potential of
Chenopodium
ambrosioides
Linn Essential Oil against
Anopheles gambiae
Giles
(
Diptera: Culicidae). The Open Entomology Journal,
7
: 16-22
Kothari C.R., 2004, Research design: research methodology, methods and
techniques. 2
nd
edition. New Age International Publishers, New Delhi,
India, 47-50
Maurya P., Mohan L., Sharma P., Batabyal L., and Srivastava C.N., 2007,
Larvicidal efficacy of
Aloe barbadensis
and
Cannabis sativa
against
the malaria vector
Anopheles stephensi
(Diptera: Culicidae). Entomological
Reserves, 37: 153-156
Mgbemena I.C., 2010, Comparative evaluation of larvicidal potentials of
three plant extracts on
Aedes aegypti
. Journal of American Science, 6:
435-440
Misganaw N., Moges S., Tadele M., Tesera M., Temesgen T., and Raja N.,
2012, Evaluation of Multi Potential Bioactive Endod,
Phytolacca
dodecandra
(L’ Herit) Berries Extracts Against Immature Filarial Vector
Culex quinquefasciatus
Say (Diptera: Culicidae). Research Journal of
Environmental and Earth Sciences, 4(7): 697-703
Mohan L., Sharma P., and Shrivastava C.N., 2006, Evaluation of Solanum
xanthocarpum extracts as a synergist for cypermethrin against
larvae of
filarial vector
Culex quinquefasciatus
(Say). Entomological Reserves,
36: 220-225


