Basic HTML Version

Journal of Mosquito Research, 2013, Vol.3, No.4, 21
-
32
ISSN 1927-646X
http://jmr.sophiapublisher.com
27
Bam
HI/
Hin
dIII and digested products were analyzed by
agarose gel electrophoresis. Figure 6 shows two bands; a
lower band of a size approximately 858 bp corresponding
to Tsf insert and an upper one of around 5 300 bp
corresponding to the linearized vector. This result indicates
a successful subcloning of insert Tsf into pET-28a vector.
The isolated 858 bp partial cDNA sequence for Tsf
encodes a deduced 248-aa peptide (Figure 7).
Figure 6 0.7% agarose gel electrophoretic analysis of
Tsf/pET-28a construct double digested with
Bam
HI/
Hin
dIII
Notes: Lane M, 1 kb DNA molecular weight marker; Lane 1,
uncut Tsf /pET-28a construct; Lane 2, digested construct
(Tsf/pET-28a) by
Bam
HI/
Hin
dIII; Lane 3, uncut pET-28a; Lane
4, digested pET-28a by
Bam
HI/
Hin
dIII
Figure 7 Partial cDNA and deduced amino acid sequence of
Cx.
quinquefasciatus
transferrin molecule
3.4 Expression and Purification of recombinant Tsf
protein
BL21 cells containing the Tsf/pET-28a construct were
induced to express 6× his-tagged Tsf recombinant protein
by adding 1 mM IPTG after the cells have reached linear
phase of growth at 37
℃
. After 4 h of induction, the cells
were collected by centrifugation and the cell pellet was
re-suspended in lysis buffer and extracted by
ultrasonication. The insoluble protein and the cell debris
were separated from the soluble proteins by centrifugation.
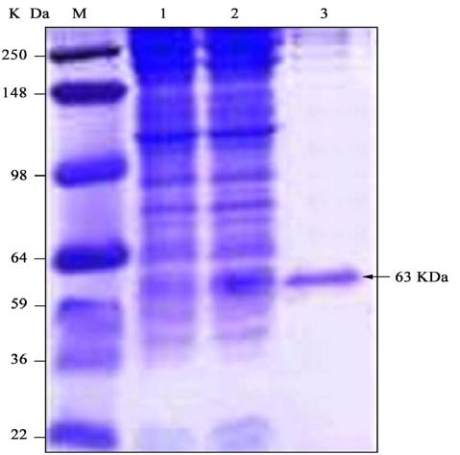
After purification, recombinant Tsf protein was obtained
in a soluble form in a relatively high purity level with a
band corresponding to 63 kDa. The total proteins from
induced and non-induced cells as well as the purified
recombinant protein were analyzed by SDS-PAGE
(Figure 8).
3.5Antimicrobial activity of the purified Tsf protein
The purified recombinant Tsf protein was tested
against a bacterial strain to prove its antimicrobial
potency. When the recombinant Tsf protein was tested
against The Mach1™- T1
®
E. coli
strain, it was found
that this
E. coli
strain is sensitive to Tsf protein as a
clear inhibition zone appeared as shown in Figure 9B.
Figure 8 Coomassie blue stained SDS-PAGE of protein extract
from transformed BL21 cells harboring Tsf/pET-28a construct
Note: The arrow points to Tsf pure recombinant protein band. Lane
M, protein molecular weight marker; Lane 1, uninducted cells;
Lane 2, cells induced with 1 mM IPTG at 37
℃
; Lane 3, Purified
Tsf recombinant protein

