Basic HTML Version

International Journal of Marine Science 2014, Vol.4, No.57, 1-4
http://ijms.biopublisher.ca
2
broken using a hammer to remove the soft body
tissue. The removed tissues were rinsed with sterile
distilled water, cut into small pieces and kept in Petri
dishes and dried at a constant temperature of 50
℃
for 24 h in a hot air oven. The dried material was
powdered thoroughly for solvent extraction (Vimala
and Thilaga, 2012).
1.2 Solvent extraction
The powdered tissues of
H. pugilinus
were extracted
with eight different solvents like methanol, ethanol,
acetone, acetonitrile, dichloro methane, chloroform,
ethylacetate and distilled water with the help of soxlet
apparatus then the solvents were concentrated with the
help rotary evaporator (VC100ALark Rotavapor® at
30
℃
) with reduced pressure to give a dark brown
gummy mass. The resultant residues were stored at
4
℃
for further antibacterial activity.
1.3 Antibacterial Attributes of
H. pugilinus
Six species of bacteria were used for the antibacterial
activity, including
V.parehaemolyticus
(J13300),
A.hydrophilla
(IDH1585),
S.typhi
(C6953),
S.paratyphi
A (C6915),
V.cholera
(IDH5439) and
E.coli
(H10407).
All the bacterial strains were clinical isolates, obtained
from the Microbial Type Culture Collection & the
Gene Bank, Institute of microbial technology,
Chandigarh, India. Pathogenic bacterial strains were
inoculated in sterile nutrient broth and incubated at
37°C for 24h. Pathogens were swabbed on the surface
of the Muller Hinton agar plates and. wells of 5 mm in
diameter were made aseptically using well cutter,
and50 μL of eight different solvent extracts of tissues
and eggs were inoculated. The stock solutions were
prepared at a concentration of 20 mg/mL. Positive
control well containing 50 μL of tetracycline
(1mg/mL) and negative control containing 50 μL of
appropriate solvents were used. The result was
calculated by measuring the zone of inhibition in
millimetres. For each concentration tested, triplicates
were maintained for the confirmation of activity.
1.4 Determination of the minimum inhibitory
concentration (MIC)
The solvent extracts of marine gastropods
H.pugilinus
which showed significant antibacterial activity were
selected for the determination of MIC. A stock
solution of 20 mg/mL was prepared and serially
diluted to obtain various concentrations between 4
mg/mL and 20 mg/mL. About 0.5 mL of each dilution
of different concentrations was transferred into a
sterile test tube containing 2 mL of nutrient broth. To
the test tubes, 0.5 mL of test organism previously
adjusted to a concentration of 10
5
cells/mL was then
introduced. A set of test tubes containing broth
alone was used as a control. All the test tubes and
control were then incubated at 37
℃
for 24 h. After
the period of incubation, the tube containing the
least concentration of extracts showing no visible
sign of growth was taken as the minimum inhibitory
concentration (Pasiyappazham Ramasamy et al.,
2013).
1.5 Statistical analysis
Data on the inhibitory effects of solvent extracts of
H.pugilinus
was analyzed by one-way analysis of
variance (ANOVA) usingSPSS-16 version software
followed by Duncun’s multiple range tests and
standard deviation. P<0.05 were considered for
describing the significant levels.
2 Results
2.1 Antibacterial Attributes
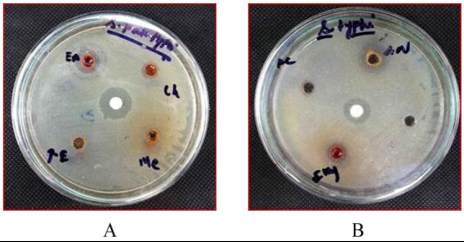
The inhibition zone in different solvent tissue extracts
of
H.pugilinus
against clinical isolate human
pathogenic bacteria was showed in Table 1. Among
H.
pugilinus
the various strains, the maximum zone of
inhibition (21.00±.0.74 mm) was recorded in Ethyl
acetate extracts against
S.paratyphi
A, followed by
(18.00± 0.84 mm) was recorded in Aceton nitrile
against
V.cholerae
and the minimum zone of
inhibition(4.00±0.51mm) was noticed in Acetone,
methanol, chloroform and water extract (Figure 1).The
positive control(tetracycline) was active against all the
bacterial strains tested.
Figure 1 Antibacterial activity of
H. pugilinus
against Human
pathogenic bacteria

