基本HTML版本

Computational Molecular Biology 2014, Vol. 4, No. 8, 1-3
http://cmb.biopublisher.ca
2
across specie and geographical locations.
Materials and Methods
A total of 22 Rabies virus sequences from 5 species
(Dog, Cat, Cow, Wolf and Fox) across 8 locations
(Nigeria, India, Ghana, Pakistan, Niger, Brazil,
Argentina and Texas) were obtained from the GenBank.
The GenBank accession nos are FJ228677.1,
FJ228678.1, FJ228681.1, FJ228683.1 (Fox): HM368163.1
(Cat), DQ105964.1 (Wolf), DQ105964.1 (Cow),
EU038108.1 EU038106.1 EU038105.1 EU038103.1,
HM368162.1 HM368160.1 FJ545679.1 FJ545678.1
FJ545674.1, FJ545678.1 FJ545674.1, DQ105963.1,
AY654585.1 JN106463.1 AY233451.1, AY233450.1
(Dog). Sequence alignments were carried out using
clusterW w (Larskin et al., 2007). A Neighbor-joining
tree on the basis of genetic distances depicting
phylogenetic relationship among Rabies viruses was
constructed using the complete deletion and p-distance
option using the MEGA VERSION 5 SOFTWARE
(Tamara et al., 2011) (Figure 1).
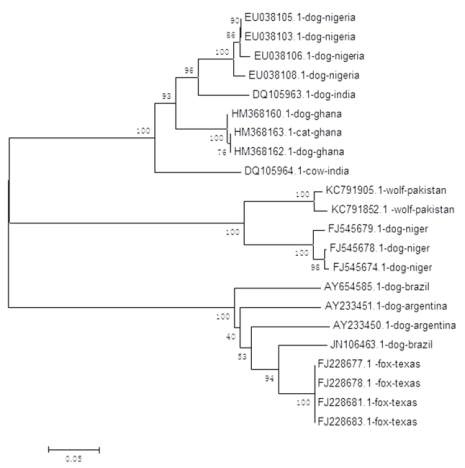
Figure 1 Phylogenetic tree derived from nucleotide sequences
of rabies viruses using the Neighbor-Joining method
Results and Discussion
The phylogenic analysis reveals a strong subdivision
of rabies viruses by geographical location (Table 1).
The phylogenic groups also form clusters associated
with species from which the virus is isolated. It has
been reported that the lyssaviruses are characterized
by their ecological association with specific
mammalian species, which act as vectors for their
transmission, such that that a number of phylogenic
lineages co-circulate among a range of mammalian
hosts (Davies et al., 2005). The phylogenic structure
may be explain by the importance of geographical
barriers to gene flow as previously demonstrated for
rabies virus in Europe (Bourhy et al., 1999). The
closest relationship is seen between virus isolates from
a dog and a Cat both from Ghana, followed by isolates
from a Dog and Cow from India (Table 2).
References
Bourhy H., Kissi B., Audry L., Smreczak M., Sadkowska-Todys M.,
Kulonen K., Tordo N., Zmudzinski J.F.,and Holmes E.C.,1999,
Ecology and evolution of rabies virus in Europe. J Gen Virol.
80:2545-2557
Davis P.L., Holmes E.C., Larrous F., Van der Poel W.H., Tjornehoj K.,
Alonso W.J., andBourhy H.,2005, Phylogeography, population
dynamics, and molecular evolution of European bat lyssaviruses. J
Virol. 79:10487-10497
Denduangboripant J., Wacharapluesadee S., Lumlertdacha B., Ruankaew
N.,and Hoonsuwan W., 2005 ,Transmission dynamics of rabies virus in
Thailand: implications for disease control. BMC Infect Dis 5: 52
Kuiken T., Holmes E.C., McCauley J., Rimmelzwaan G.F., Williams
C.S.,and Grenfell B.T., 2006,Host species barriers to influenza virus
infections. Science. 312:394-397
LarkinM.A.,2007 ,ClusterW and Clusterx version. Bioinformatics,
23:2947-2948
Tamura K., Peterson D., Peterson N., Stecher G., Nei M., and Kumar S.,
2011) ,MEGA5: Molecular Evolutionary Genetics Analysis using
Maximum Likelihood, Evolutionary Distance, and Maximum
Parsimony Methods. Molecular Biology and Evolution 28: 2731-2739
WHO Expert consultation on rabies:first report,2005
Wunner W.H., Larson J.K., Dietzschold B.,and Smith C.L., 1988 ,The
molecular biology of rabies viruses. Rev Infect Dis 10 Suppl 4:
S771-784

