Basic HTML Version

Tree Genetics and Molecular Breeding 2014, Vol.4, No.2, 1
-
10
http://tgmb.biopublisher.ca
6
browning, the fact that QTLs for the two types of
browning were located on the same linkage group
suggests that fruit flesh and fruit juice browning are
controlled by similar mechanisms. A major QTL for
fruit acidity, presumably
the
Ma
locus, was detected
on the upper part of LG 16 in ‘Fuji’ as described in
previous studies (Kenis et al., 2008; Liebhard et al.,
2003; Xu et al., 2011). Interestingly, this QTL was
tightly linked to the fruit juice browning QTL (Figure
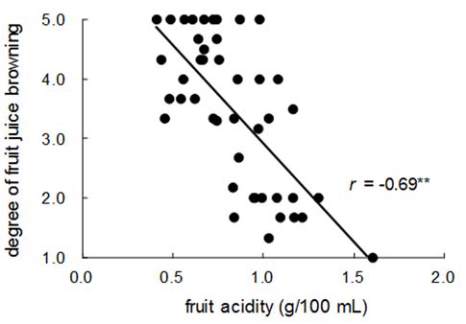
2); in addition, a high correlation (
r
= −0.69**) was
found between the two traits (Figure 3 and Figure 4).
Those result may provide preliminary data, however,
because limited number of genotypes produced fruits
in each year (n=45 to 67). The reason why phenotypic
variance explained by the QTL on LG16 fluctuated
among years is probably due to the variable number of
individuals (n=45 to 67) that fruited in each year. A
small number of fruited progeny could result in a
distorted frequency distribution, and may cause an
overestimate of total phenotypic variance in QTL
analysis.
Figure 4 Correlation between degree of fruit juice browning
and fruit acidity in F
1
populations of ‘Fuji’בMaypole’
Note: **: Significant at
p
=0.01
In the area around the QTL region for both fruit juice
browning and fruit acidity, six progeny were identified
as recombinants with crossover between SSR markers
CH02a03 and CH05c06. Genotyping of recombinants
with newly designed SSR markers delimited the QTL
to a physical size of 514 kb sandwiched by
LG16-1159074 (1.16 Mb) and LG16-1661111 (1.67
Mb) (Figure 5a; Figure 5b). Fine genetic mapping of
the
Ma
locus has recently narrowed down the
Ma
region to 1.30–1.43 Mb (Xu et al., 2011), consistent
with our results. Our study, however, is the first to
physically identify the QTL controlling fruit juice
browning in apple.
Among the 105 genes predicted within the 514 kb
region, ALMT has been identified as a strong
Ma
candidate because its expression is significantly
correlated with fruit titratable acidity (Bai et al., 2012).
Furthermore, the cited researchers identified a
mutation at 1,455 bp leading to a premature stop
codon that truncates the carboxyl terminus of the
ALMT protein; the SNP was completely associated
with low fruit acidity, suggesting that the natural
mutation-led truncation is most likely responsible for
the abolished function of
Ma
for high fruit acidity in
apple.
Although candidate genes for fruit juice browning
have not been identified in apple, fruit flesh browning
QTLs and their association with candidate genes
encoding PPO (LG10) and PAL (LG4, 12) have been
reported (Guardo et al., 2013). In apple, susceptibility
to flesh browning is thought to be the result of
complex interplay between the PPO enzyme and
polyphenol content (Amiot et al., 1992). The PPO
enzyme catalyzes the formation of quinones from
polyphenols such as chlorogenic acid and catechin,
resulting in browning of fruit flesh and juice (Boss et
al., 1995; Falguera et al., 2011). PAL is a key enzyme
of the phenylpropanoid pathway that catalyzes the
deamination of phenylalanine to trans-cinnamic acid,
the latter a precursor to chlorogenic acid, catechin, and
anthocyanin (MacDonald and D’Cunha 2007).
Although the two candidate genes reported by Guardo
et al. (2013) are not located within the QTL region of
chromosome 16, several hypotheses concerning PPO
activity and polyphenol content can be advanced
based on the candidates within the region. First, PPO
activity might be affected by fruit acidity. Malic acid
content, the main determinant of fruit acidity in apple,
is strongly correlated with fruit juice pH, and QTLs
for fruit acidity and pH co-locate on the
Ma
locus (Bai
et al., 2012; Xu et al., 2011; Morimoto et al.,
unpublished data). Fruit pH appears to be associated

