Bt Research 2015, Vol.6, No.3, 1-10
4
Figure 3B Jisha et al. (Color in web edition)
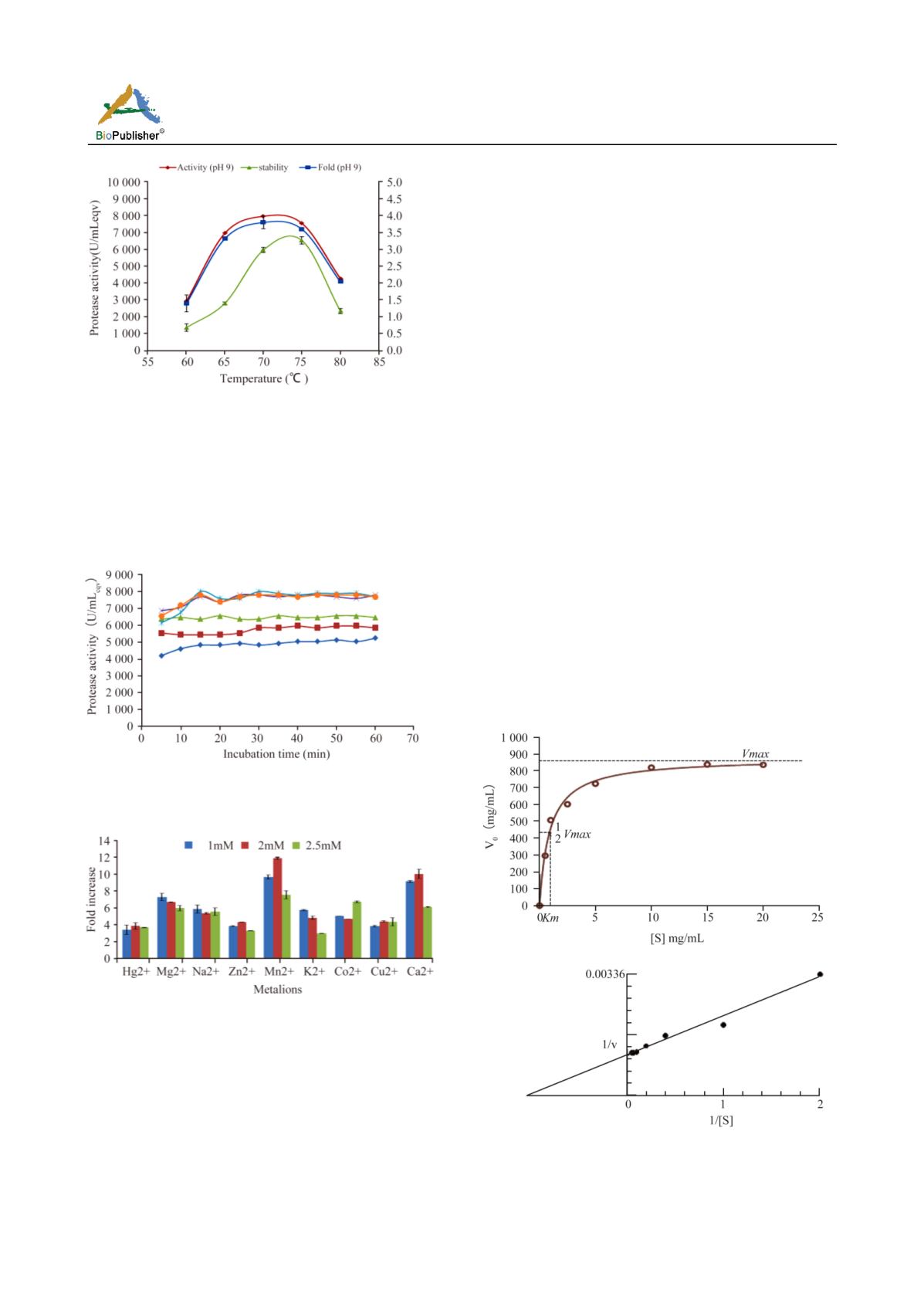
3B.Optimisation of temperature and thermal stability of alkaline
protease. Effect of temperature on the protease activity was
determined by incubating the reaction mixture at different
temperatures (60-80
℃
) with pH 9 and 20 min incubation using
10 mg/mL casein. For thermal stability the protease was
pre-incubated at different temperatures (60-80
℃
), for 1h and
then activity was assayed.
Figure 3C Jisha et al. (color in web edition only)
3C Effect of substrate concentration on the protease activity
Figure 3D. Jisha et al. (color in web edition only)
3D. Effect of metal ions (mM) on protease activity. This
activity was at 70
℃
, 9.0 pH with 15 mg/mL casein for 30 min
incubation.
incubation for 30 min; which was 3.8 folds increase
over the initial activity
(Figure 3C).
Effect of metal ions on protease activity
Effect of metal ions (salts) on protease activity was
studied under the assay conditions: 15 mg/ml (casein)
substrate at 70
℃
, pH 9 with incubation for 30 min.
The protease activity was enhanced with the addition
of Mn
2+
, Ca
2+
or Mg
2+
, and the maximum activity
(11732 U/ml
eqv
) was obtained in the presence of 2 mM
Mn
2+
,
which was 5.6 folds increase over the initial
activity (Figure 3D).
Thus, the
optimized conditio
n for the activity of
protease from
Btk
was: 15 mg/ml (1.5%) casein as
substrate, 2 mM Mn
2+
, pH 9.0 and 70
℃
temperature
for 30 min incubation.
Enzyme kinetics
The
Km
and
Vmax
values for
Btk
protease were calculated
using the data obtained for different substrate
concentration. The
Km
and
Vmax
values were found to
be 0.90 mg/ml and 879.3 U/mg, respectively (Figure 4).
Protease inhibition and response to detergents
Protease inhibition was studied by adding EDTA, the
chelating agent and protein de folding agent
β
-mercaptoethanol in the reaction mixture. The
optimized reaction condition was employed for this
experiment. Of the complex compounds, presence of
1mM EDTA or
β
-mercaptoethanol in the reaction
mixture decreased the protease activity to 6% (1972
Figure 4 Jisha et al. (Color in web edition only)
Nonlinear fit of Michaelis-Menten data. (Effect of substrate
concentration on alkaline protease activity) and Line
weaver-Burk plot


