Bt Research 2015, Vol.6, No.3, 1-10
3
Table 1 Summary of purification of extracellular protease secreted by
Btk
from the supernatant in LB medium supplemented with
30% (w/v) raw soybean flour
Purification
Total protein (mg) Total activity (U/mL
eqv
) Specific activity (U/ mg protein) Yield (%) Fold
Crude extract
17.21 ±0.92
2465.16 ±4.35
138.027
100
1
60-80 % (NH
4
)
2
SO
4
Fraction 9.06 ±0.40
2387.3 ±7.24
271.90
0.968
1.97
Spin column Fraction
5.29 ±0.93
2213.11 ±28.97
371.95
0.897
2.7
Sephadex G-100 column
Fraction
0.46 ±0.05
741.8 ±11.59
1766.2
0.3
12.8
Figure 2 Jisha et al. (Color in wb edition only)
2.
Sephadex G-100 elusion profile of the partially purified
protease. (NH
4
)
2
SO
4
fraction (60-80%) of crude protein
harvested from soybean flour supplemented (30%, w/v) LB at
12h fermentation was subjected to MW cut-off using Vivaspin
6 spin column, and the lower fraction was loaded on sephadex
G-100, which was eluted using phosphate buffer (pH 7.6) with
a flow rate of 1.5 mL per 10 min.
protease purified from the supernatant obtained from
this modified LB medium was used for further studies.
The initial activity of protease is the product of
activities of Sephadex G-100 gel filtration fraction
minus that of LB control.
Characteristics of Protease
Effects of pH, temperature, substrate and metal ion
concentrations were tested to fix the optimum activity
of the purified protease (active fraction obtained by
Sephadex G-100 gel filtration).
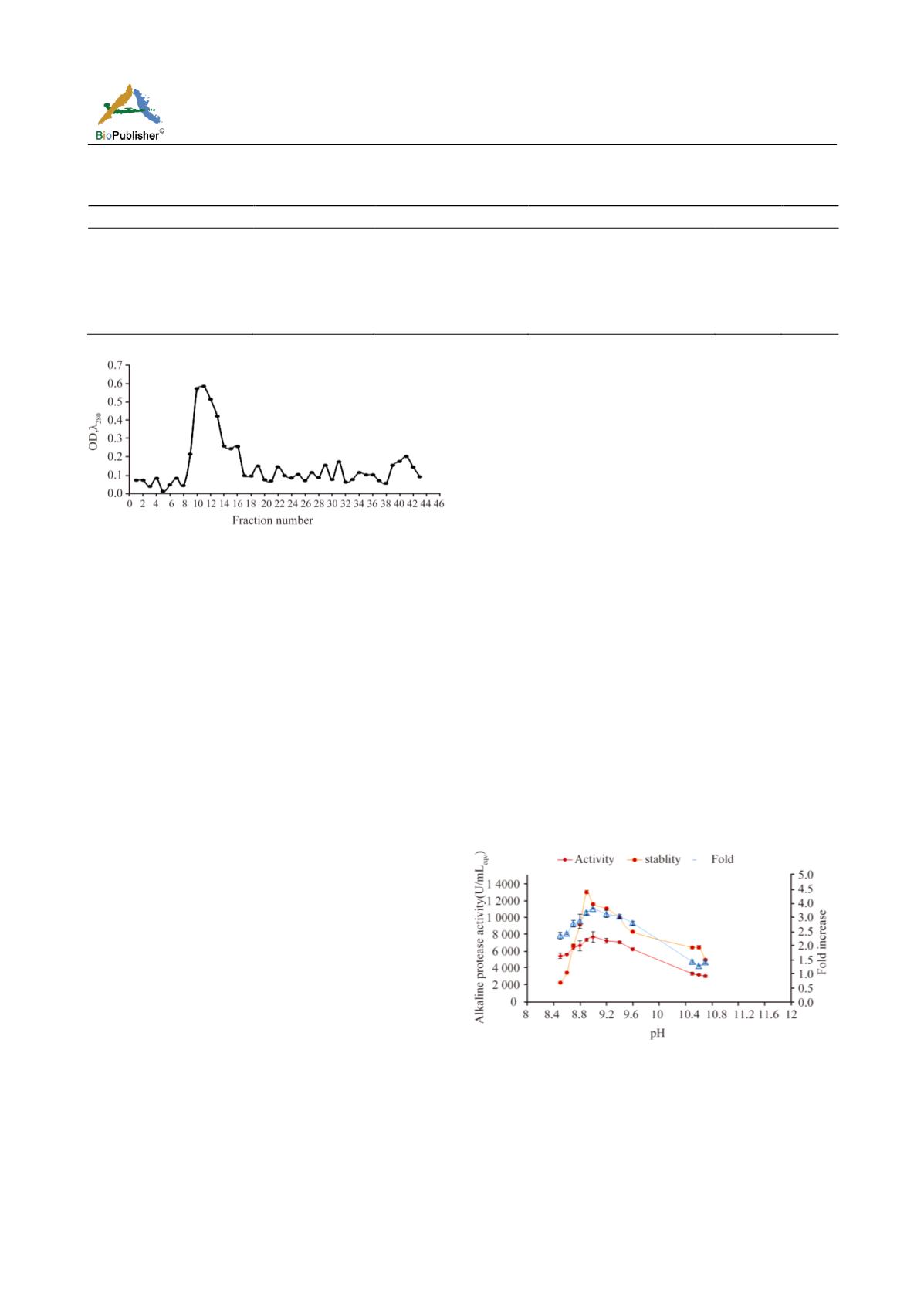
Effect of pH
For estimating the pH optimum, the concentration of
substrate (10 mg/ml casein), temperature (37
o
C) and
incubation period (20 min) were fixed as used for
determining initial activity. At these conditions, the
protease showed better activity in the range 8.5 to 11.5,
with optimum activity at pH 9 (7684.4 U/m
eqv
), which
was 3.7 folds increase over the initial activity.
Interestingly, another peak of protease activity was
also obtained at pH 11 with 3.1 folds increase (6455
U/ml
eqv
) over the initial activity (Figure 3A). Thus, the
protease showed broad range stability on the alkaline
side. However, for further characterization studies,
pH9 was fixed.
Effect of temperature
For estimating temperature optimum, the concentration
of substrate (10 mg/ml casein), pH (pH9) and
incubation period (20 min) were fixed as used for
determining initial activity. At these conditions, the
protease showed better activity (7923 U/ml
eqv
)
at
70
℃
(Fig. 3B), which was 3.8 folds increase over the
initial activity, with comparable activities at 65
℃
and
75
℃
. Protease was more or less stable up to 80
℃
. At
pH 11, the protease showed the maximum activity
(7633 U/ml
eqv
) at 75
℃
(Figure 3B);
thus,
the existence
of two temperature optima further indicates that there
exist two proteases.
Effect of substrate concentration
Effect of substrate concentration (casein, 0.5, 1, 5, 10,
15, 20 mg/ml) was measured at 70
℃
pH-9 and 20
min incubation,
i.e
., 0.05 to 2%. The maximum activity
was 7992 U/ml
eqv
noticed with 15 mg/ml casein at an
Figure 3A. Jisha et al.( Color in web edition only)
3A. Optimization of pH, fold increase over initial activity and
pH stability of alkaline protease. Effect of pH on protease
activity was studied at 37
℃
, varying pH, 10 mg casein as
substrate for 20 min incubation (30% Soybean flour supplemented
LB after 12 h fermentation). Stability of the enzyme at various
pH values was assayed by pre-incubating the enzyme in buffers
of different pH for 1h.


