Basic HTML Version
International Journal of Clinical Case Reports
77
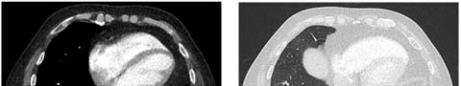
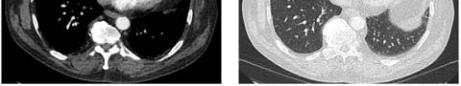
Figure 2 The images above shows complete resolution of the
PAVM. Only a subtle residual fibrosis of lung tissue was seen
at the right lower lobe
The patient was followed up in the out-patient
department and his symptoms have completely
resolved. He was thus discharged from the
cardiothoracic department.
2 Discussion
Pulmonary arterio-venous malformation (PAVM) is a
rare vascular lesion which accounts for 2-3 per 100
000 population (Hayashi et al., 2012; Dewar and
Schonell, 1966). PAVM is classified into primary and
secondary PAVM, also known as acquired
pulmonary AVM.
The aetiology of primary vascular abnormality is
unknown but it is strongly associated with Hereditary
Haemorrhagic Telangiectasia, which accounts for
almost 70% of the incidence of PAVM (Ghersin et al.,
2010). Secondary PAVMs are rare. Reported causes
include chest trauma, long standing hepatic cirrhosis,
metastatic lung carcinoma ad amyloidosis (Kurshid
and Downie, 2002; Prager et al., 1983; Symbas et al.,
1980; Kamei et al., 1989; Pierce et al., 1959).
Majority of the patient with PAVM are asymptomatic.
The discovery of PAVM is usually through routine
chest x-ray (Kurshid and Downie, 2002). The classical
triad of presentation is dyspnea, cyanosis and clubbing
(Prager et al., 1983). In patients with Osler-Weber-Rendu,
presentation such as recurrent epistaxis and cardiac
murmur may also be present (Dewar and Schonell,
1966). Chest x-ray is a valuable tool in detecting the
vascular lesion. It is also commonly used for
clinical follow up (Kurshid and Downie, 2002;
Sluiter-Eringa et al., 1969) Computed Tomography
(CT) Scan, especially a contrast enhanced CT scan
is good in defining the vascular lesion (Kurshid and
Downie, 2002).
There have been no reported cases of spontaneously
resolved inflammatory AVM in the literature. We are
the first team to report this. From this experience, we
suggest that in a spontaneously occurring
inflammatory PAVM, conservative management
should be adapted in the first instance instead of
putting patients through surgery to begin with. Close
monitoring of the condition should be initiated to
confirm
resolution.
Surgical
excision
and
endovascular embolization has been the treatment of
choice for PAVM (Hayashi et al., 2012; Kurshid and
Downie, 2002). However, there has been no similar
case being reported. Hence, it is difficult to ascertain
the best way of management forward. More relevant
cases should be reported to compare management
options and prognosis.
3 Conclusion
Even though the treatment for arterio-venous
malformation is surgical resection, the approach for an
inflammatory AVM can be taken conservatively
because it can spontaneously resolve.
However, close monitoring is essential to ensure
complete resolution.
If there is no complete resolution, either surgical or
endovascular intervention might be needed.
Authors’ contribution
Wong KB – main author; Chan SA – contribute in
writing the discussion; Abid Q – Final approval of
the script.
References
Dewar V., and Schonell M., 1966, Hereditary haemorrhagic
telangiectasia with pulmonary arterio-venous fistulae,
Postgrad Med J., 42(493): 728-730
http://dx.doi.org/10.1136/pgmj.42.493.728
Ghersin E., Hildoer D.J., and Fishman J.E., 2010, Pulmonary
arteriovenous fistula within a pulmonary cyst – evaluation
with CT pulmonary angiography, Br J Radiol., 83(990):
e114-e117
http://dx.doi.org/10.1259/bjr/39651947
Hayashi S., Baba Y., Senokuchi T., and Nakajo M., 2012,
Efficacy of Venous Sac Embolization for Pulmonary
Arteriovenous Malformations: Comparison with Feeding
Artery Embolization, Journal of Vascular and
Clinical Case Reports, Int’l Journal of

