基本HTML版本

International Journal of Horticulture 2014, Vol.4, No.15, 1
-
4
http://ijh.biopublisher.ca
4
Table 3 Skeletal ANOVA: Number of single node cuttings obtained from seven potato cultivars in three consecutive subculture
intervals in season 2
Source of variation
Degrees of freedom
MS
F.pr.
Rep
2
9.125
Potato cultivar
6
228.598
<.001
Error a
12
9.461
Subculturing cycle
2
271.949
<.001
Potato cultivar x subculturing cycle
12
13.457
0.043
Error b
28
6.151
Total
629
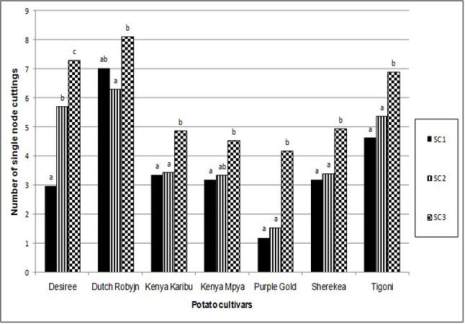
the first two subculture cycles. In addition, Purple
Gold gave the least number of single node cuttings
(2.300) while Dutch Robyjn gave the highest (7.144).
Figure 2 Number of single node cuttings obtained from seven
potato cultivars over three consecutive subculture intervals in
season 2
Note: Within a potato cultivar, means sharing the same letter
are not significantly different from each other at P≤ 0.05. SC
1= subculture cycle 1, SC 2= subculture cycle 2, SC 3=
subculture cycle 3
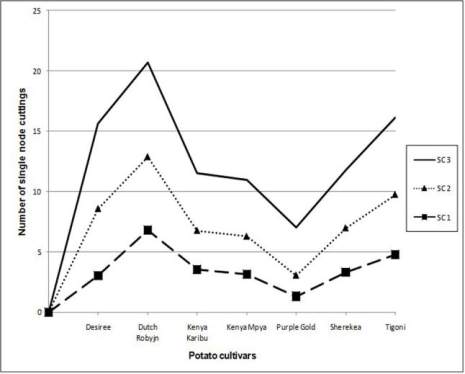
All the potato cultivars followed a similar trend in
production of single node cuttings (Figure 3). In all
cases, the third subculture interval gave more cuttings
than the first two.
Differences in the number of single node cuttings
generated from potato cultivars could be due to genetic
differences among the potatoes; the harder and woody
the potato stem, the slower the generative rate and the
fewer the number of single nodes generated. Differences
in the number of single node cuttings generated in
different subculturing cycles could not be explained.
References
Badoni, A. and J. S. Chauhan. 2010. Conventional vis -a- vis biotechnological
methods of propagation in potato: A review. Stem Cell 1: 1-6
Kassanis, B. 1957. The use of tissue cultures to produce virus-free clones
from infected potato varieties. Ann. Appl. Biol 45: 422-427
Figure 3 Average number of single node cuttings obtained from
seven potato cultivars over three consecutive subculture
intervals across the two seasons
Note: SC 1= subculture cycle 1, SC 2= subculture cycle 2, SC
3= subculture cycle 3
Murashige, T. 1974. Plant propagation through tissue culture. Annual
Review of Plant Physiology 25: 135-166
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and
bioassays with tobacco tissue cultures. Physiol. Plant 15: 473-497
Naik, P. S. and J. L. Karihaloo. 2007. Micropropagation for the production
of quality potato seed in Asia-Pacific. Asia-Pacific Consortium on
Agricultural Biotechnology. New Delhi, India
Ng, S. Y. C., G. Thottapily, and H. W. Rossel. 1992. Tisue culture in disease
elimination and micropropagation. p. 171-182. In G. Thottapilly, L.M.
Monti, D.R. Mohan Raj and A.W. Moore(ed) Biotechnology: Enhancing
Rsearch on Tropical Crops in Africa. CTA/IITA co-publication.
International Institute of Tropical Agriculture, Ibadan, Nigeria
Payne, R. W., D. A. Murray, S. A. Harding, D. B. Baird, and D. M. Soutar.
2011. GenStat for Windows 14th ed. Introduction. VSN International,
Hemel Hempstead
Steel, R. G. D. and J. H. Torrie. 1980. Principles and procedures of statistics:
A biometrical approach. 2nd ed. McGraw-Hill, New York
Waithaka, K. 1988. Application of plant tissue and cell culture in
horticultural production. Acta Horticulturae 218: 131-139
Waithaka, K. 1992. Micropropagation techniques and the production of
pathogen-free plants. p. 183-187. In G. Thottapilly, L.M. Monti, D.R.
Mohan Raj and A.W. Moore (ed) Biotechnology: Enhancing Rsearch
on Tropical Crops in Africa. CTA/IITA co-publication. International
Institute of Tropical Agriculture, Ibadan, Nigeria
Wang, P. J. and C. Y. Hu. 1980. Regeneration of virus-free plants through in
vitro culture.p. 61-99. In A. Flechter (ed.) Advances in biochemical
engineering. Springer-Verlag, Berlin

