International Journal of Aquaculture, 2017, Vol.7, No.4, 23
-
30
28
efficiency depressed when diet contains 15% or more lipids (Kamalam et al., 2017). Rainbow trout has an
exclusive requirement of n-3 or w3 PUFA in their diet. High protein level in the diet is always resulted an increase
in unwanted ammonia excretion. It was observed that diet with 45% and 40 % protein level resulted as 0.12 mg/l
and 0.08 mg/l ammonia excretion in the drain water, respectively. But, lowest level of 0.05 mg/l ammonia was
recorded with 35% protein level in the diet.
A major researchable issue for trout feeding is the high cost of manufactured pelleted feed due to the use of
largely imported fish meal. The increasingly scarce supply of fish meal and its high market price had made it
necessary to seek a cost effective replacement of fish meal to supply dietary protein in trout feed. For addressing
the issue, two cost - effective practical starter feeds were formulated for first feeding rainbow trout fry, based on
single or multiple protein sources. The experimental diets were made up of commercial ingredients and were
conventionally prepared by steam cooking, pelleting and drying. To evaluate the performance of these two diets as
compared to a widely used commercial diet, a five week feeding trial was conducted and found more efficient and
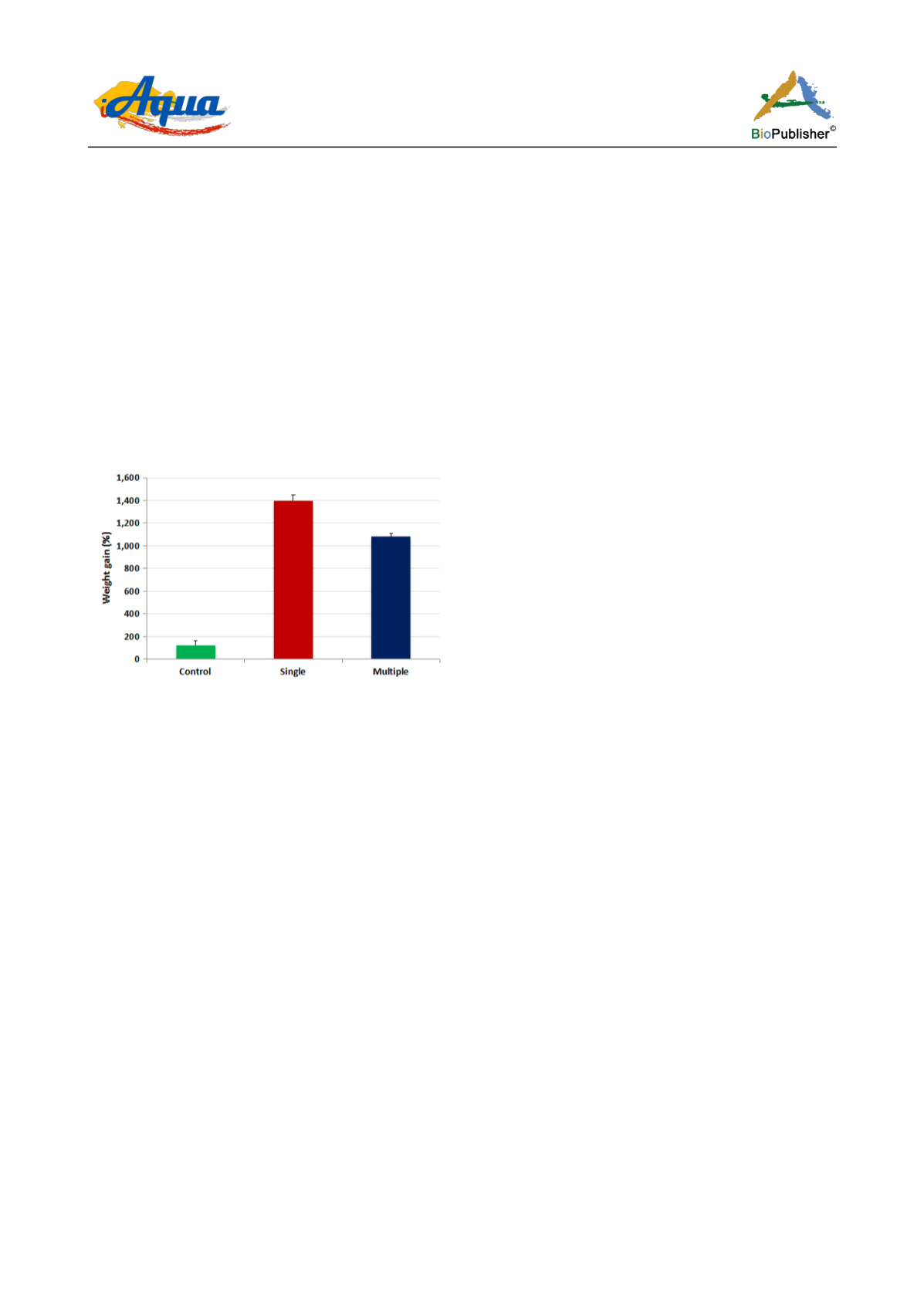
significant over the commercially used starter feed (Figure 3).
Figure 3 Experimental results of ICAR - DCFR formulated trout feed on growth
D. Health Management
Pathogenic bacterial profiling of rainbow trout farms of Himachal Pradesh, Uttarakhand and Sikkim was
conducted. Major pathogenic bacteria characterized from the samples were
Citrobacter freundii, Pantoea
agglomerans, Aeromonas hydrophila, Aeromonas veronii, Hafnia alvei, Pseudomonas
fluorescens, Plantibacter sp
,
Morganella sp, Staphylococcus sp, Plegiomonas sp, Carnobacterium maltaromaticum & Corynebacterium sp.
Surveillance of rainbow trout farms at different locations observed bacterial diseases like tail rot, gill rot, fin rot
and exophthalmia. Skin hemorrhagic ulcers on the caudal regions of rainbow trout were also observed,
characterized by deep ulcers and hemorrhagic frayed fins. The causative agent was identified as
Aeromonas
hydrophila
(designated RTMCX1). Amplification and sequencing of the 16S rRNA gene (1 304 base pair)
revealed that the sequence was 99% similar to
A.
hydrophila
sub sp.
hydrophila
. This strain exhibited strong
ß-hemolytic activity against sheep blood and rainbow trout erythrocytes, and was capable of growing in rainbow
trout serum and 0.5 - 10% NaCl at 4.0 - 35°C. Mortality was 100% in experimentally-infected fish, with a median
lethal dose (LD50) of 1.9 × 10
4
colony forming units/g body weight.
Aeromonas hydrophila,
RTMCX1 was
sensitive to a majority of 27 tested antibiotics, indicating the possibility of controlling this bacterium by
antimicrobial compounds. Four
strains of
Lactococcus garvieae
(GenBank accession no: KM 604701, KM 604702,
KM 604703 and KM 604704) were isolated and identified from diseased rainbow trout in trout farms of
Uttarakhand and Himachal Pradesh. Samples were showing typical symptoms of lactococcosis; unilateral
exophthalmia, inflammation and swollen vent, petechial haemorrhages in viscera, focal haemorrhages and
swelling in liver (Shahi et al., 2013; Mallik et al., 2016). Further, disease surveillance of trout farms was
conducted in Himachal Pradesh and Jammu & Kashmir under National Surveillance Programme on Aquatic
Animal Diseases (NSPAAD). So far a total of 25 fish farms from three states namely, Jammu and Kashmir,
Himachal Pradesh as well as Uttarakhand were surveyed. Screening of coldwater fish viruses i.e., infectious


