Basic HTML Version


Bioscience Methods
BM 2011, Vol.2, No.4, 21-30
http://bm.sophiapublisher.com
- 24 -
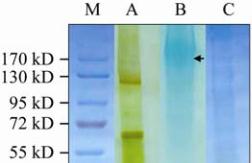
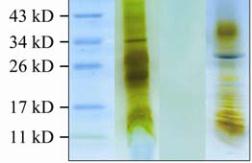
Figure 2 SDS-PAGE analysis of jellyfish mucin
Note: The relative molecular mass and purity of mucin samples
were determined using SDS-PAGE; The proteins were resolved
by gradient 6%~18% acrylamide gels, visualised by first
staining with silver followed by a counter-stain with 1% Alcian
Blue; A: molecular weight standards; B: bell protein extract
following dialysis (supernatant 2 in Figure 1A); C: purified bell
mucin after tryptic treatment and HIC purification; D: jellyfish
exudate mucin after tryptic digestion; The arrow indicates
diffuse blue staining mucin bands
that there is substantial O-linked glyco- sylation with
only this monosaccharide. The presence of sialic acid
is consistent with the alcian blue staining of the mucin
and its poor affinity for hydrophobic interaction
chromatography. The presence of smaller amounts of
N-acetylglucosamine (GlcNAc) and mannose may
indicate the existence of either more complex
O-glycans or a small quantity of N-linked
oligosaccharides. The amino acid composition of the
bell mucin was heavily biased with five amino acid
residues. Indeed, the sum of Thr, Ala, Val, Pro and Glu
residues accounted for 93% of all amino acids (Table 1).
An abundance of Thr, Ala, Pro and Glu residues is
typical of most mucins (Chen et al., 2008). The
approximate equimolar quantities of Thr, Ala, Val and
to a lesser extent Pro suggests repetitive tetrapeptide
or pentapeptide (if Glu is also included) core
structures. This biased composition is also consistent
with the tandem octapeptide amino acid composition
present in Q-mucin (Masuda et al., 2007). The minor
amounts of Lys and Arg reflected the resistance of this
mucin to trypsin digestion.
1.3 Inhibition of bacterial adhesion
To test for bacterial adhesion inhibition activity of the
jellyfish mucins, we carried out experiments using the
human corneal epithelial (HCE) cell line grown in cell
culture and an ocular isolate of
Pseudomonas
aeruginosa
(Paer6264-GFP). Table 2 shows the effects
of bell mucin, exudate mucin and bovine MUC1 on
the adhesion of Paer6294-GFP to HCE cells following
preincubation of the mucins with the bacteria. Bell
mucin was the strongest inhibitor of bacterial binding
to HCE cells compared to exudate mucin and bovine
MUC1. The latter mucin was prepared from bovine
milk and has been previously shown to interfere with
the binding of bacteria to various animal cells grown
in culture (Parker et al., 2010). The number of adhering
bacteria was
reduced from 10.5×10
5
cells in the
control to 1.4×10
5
cells when 100 μg/mL of bell
mucin was used in the assay, i.e. 86% inhibition. The
result also suggested that bell mucin has more
anti-bacterial adhesion activity than bovine MUC1.
The exudate mucin showed both the rheological
properties of mucus (data not shown) and considerable
inhibition (26%~55%) of bacterial binding to HCE
cells (Table 2).
Sugars attached to the mucins are the likely candidates
binding bacteria and preventing their attachment to
HCE cells. Therefore we tested whether the major
monosaccharides found in
C. mosaicus
bell mucin
could modulate bacterial binding to HCE cells. When
GalNAc, Gal and Glc were used at varying
concentrations in the bacterial adhesion assay, both
GalNAc and Gal, were able to inhibit bacterial
adhesion to HCE cells (Table 3). Binding of bacteria
to HCE cells was not affected by Glc.
2 Discussion
In this study we isolated bell mucin from the blue
blubber jellyfish,
C. mosaicus
,
using a combination of
trypsin resistance to digestion and hydrophobic
interaction chromatography.
C. mosaicus
bell
mucin
has high molecular mass (120~300 kD) with
oligosaccharides contributing more than 50% of the
molecular mass of the glycoprotein (Table 1 and
Figure 2). Amino acid and monosaccharide compositional

