Basic HTML Version


Molecular Plant Breeding Provisional Publishing
Molecular Plant Breeding 2012, Vol.3, No.4, 37
-
49
http://mpb.sophiapublisher.com
46
chitinase and
Ag-Afp
protein from
Aspergilos gigenteous.
Our results indicate that chitinase and chitosanase
proteins from
T. harzianum
worked in collaboration
with endogenous plant defence response system and
brought the transgenic to advantage as initially there
was not a big difference in the number of developing
colonies but the difference became visible afterwards
in the health and size of colonies. If we talk about
constitutive and inducible expression, it did not look
to a major factor. Bieri et al.,
(2003) showed no
reduction to less reduction in wheat susceptibility to
E.
graminis
by high overexpression of RIP. Bieri et al.,
(2003) overexpressed barley seed antifungal proteins
in wheat and checked the effect separately of alone
chitinase, β
-
1,3
-
glucanse, RIP and Barnase as well as
in combinations. RIP transgenics showed maximum
reduction in powdery mildew susceptibility while
chitinase and β
-
1,3
-
glucanse combination showed
different levels of increase or decrease in susceptibility.
A combination of three antifungal genes
i.e.
chitinase,
RIP and β
-
1,3
-
glucanse produced by crossing did
never show reduction of susceptibility better than the
best parent. The little increase in the susceptibility of
the lines
I.A
-
8
and
I.A
-
9
indicate that it is not
necessary to quantitatively increase the anti-fungal
proteins to increase resistance against powdery
mildew disease rather a basal provision of anti-fungal
proteins either produced constitutively or induced
helps to increase disease resistance by working with
endogenous plant defence system (Bieri et al.,
2003). Sometimes this interaction does not bear
results as is seen for lines
I.A
-
8
and
I.A
-
9
and seen
by Bieri et al (2003).
3 Materials and Methods
3.1 Wheat transf rmation
For wheat transformation winter wheat genotype
‘Florida’ was selected. This genotype was maintained
under standard conditions described by Leckband and
Loerz, 1998, Becker et al., 1994 and Oldach et al.,
2001. Tissue culture media, particle preparation, DNA
precipitation and bombardment protocols was also
followed from them. This genotype is highly responsive
to tissue culture and readily transformable.
3.2 Expression vector construction
In total five expression cassettes were used in this
study. Two of them containing
HarChit
and
HarCho
separately under the control of constitutive promoter
Ubi1 from maize (Christensen et al., 1992) and
Nopalin Synthase terminator from
Agrobactrium
tumefaciens
were already cloned in the lab of Dirk
Becker. Two of the expression vectors containing
HarChit
and
HarCho
separately were cloned stress
cum disease inducible
promoter from
Vitis vinefera
L.
(Leckband and Loerz, 1998). In order to enhance the
transcriptional activity of the
Vst
-1 promoter an
enhancer fragment from the 35-S CaMV promoter was
cloned 4 times at the 5′ end of
Vst
-
1 promoter
(Serazetdinova et al., 2005). T
nos
was used as
terminator. HarChit and HarCho were amplified from
pUbiHarChit
and
pUbiHarCho
plasmids using the
primers given in table1.Vst1 and 4x Enhancer of
35
-
S promoter of CaMV were digested from
pVst1EPG
(Serazetdinova et al., 2005) plasmid,
already present in the lab. All the five vectors are
shown diagrammatically in Figure 7, Figure 8 and
Figure 9.
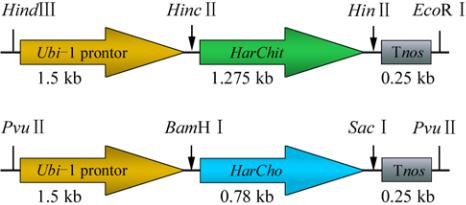
Figure 7 Vectors containing genes of interest under constitutive
promoter
Note:
HarChit
and
HarCho
under the Ubiquitin-1 promoter and
nos terminator
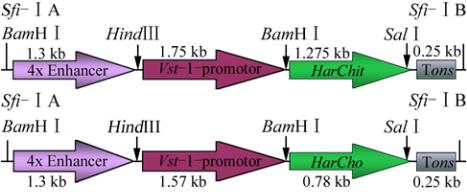
Figure 8 Vectors containing genes of interest under stress
inducible promoter
Note:
HarChit
and
HarCho
separately under the
Vst-1
promoter
and nos terminator. 4x Enhancer from CaMV, 35S Promoter is
cloned on the upstream of
Vst-1
to improve its expression level

