Basic HTML Version



Genomics and Applied Biology
, 2013, Vol. 4 No.1 1-7
http://gab.sophiapublisher.com
- 3 -
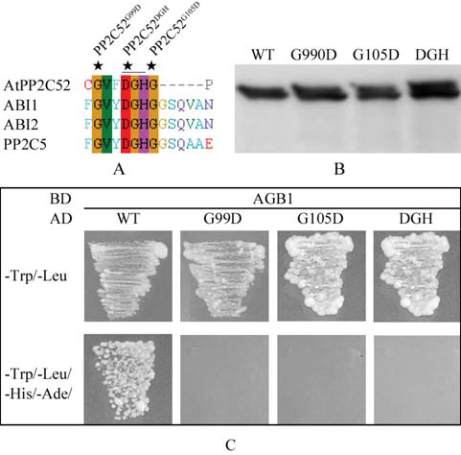
Figure 3 Site-directed mutations abolished the interaction
between AtPP2C52 and AGB1
Note: A: AtPP2C52, ABI1 (At4g26080), ABI2 (At5g57050),
and PP2C5 (AT2G40180) possess two uniquely conserved G
residues around the DGH (underlined) active site; B: Proteins
were expressed by TNT Quick Coupled Transcription/
Translation Systems and separated by SDS/PAGE, Anti-HA
antibody was used for western blot; C: Y2H analysis; When the
reporter genes were activated in the condition that the baits
(BD) interacted with the pray (AD), yeast cells can grow on
quadruple dropout medium (SD/-Ade/-His/-Leu/-Trp); WT:
Wild-type AtPP2C52:; G99D: AtPP2C52
G99D
; G105D: At-
PP2C52
G105D
; DGH: AtPP2C52
DGH102-104ERN
1.4 Potential substrates of AtPP2C52
To further identify compartments of the signaling
pathway mediated by AtPP2C52, full-length of
AtPP2C52 was used as the bait in Y2H screening.
Even on high-stringency selection media, more than
2500 positive clones were obtained and 300 clones
were sequenced. Among them, a proteasome matu-
ration factor, UMP1, and a cysteine proteinase, RD21a,
were used for further analysis.
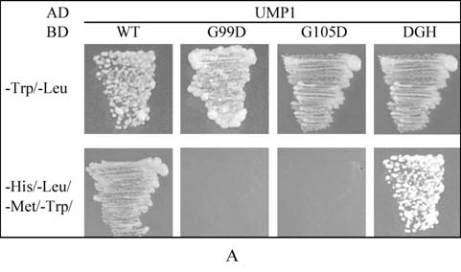
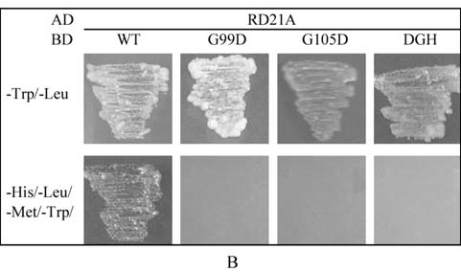
The interaction of AtPP2C52 with either UMP1 or
RD21a was confirmed by Y2H (Figure 4).
AtPP2C52
G99D
and AtPP2C52
DGH102-104ERN
mutants
failed to interact with UMP1 in Y2H (Figure 4A).
G105D mutation did not affect the Y2H interaction
between AtPP2C52 and UMP1 (Figure 4A). All of
these mutations abolished the Y2H interaction
between AtPP2C52 and RD21a (Figure 4B). The
interactions were examined by a BiFC assay in
Arabidopsis protoplast.
Figure 4 AtPP2C52 interacted with UMP1 and RD21a in Y2H
Note: When the reporter genes were activated in the condition
that the baits (BD) interacted with the pray (AD), yeast cells
can grow on quadruple dropout medium (SD/-Ade/-His/-Leu/
-Trp); WT: Wild-type AtPP2C52; G99D: AtPP2C52
G99D
;
G105D: AtPP2C52
G105D
; DGH: AtPP2C52
DGH102-104ERN
The ORFs of
AGB1, UMP1
and
RD21a
were fused
downstream of the nYFP and the ORF of
AtPP2C52
was fused upstream of the cYFP. BiFC signals of
nYFP-fused AGB1 and cYFP-fused AtPP2C52 were
detected in the peripheral region of Arabidopsis
mesophyll protoplasts (Figure 5) as previously
described (Tsugama et al., 2012a). BiFC signals of
nYFP-fused UMP1 and cYFP-fused AtPP2C52 were
also detected in the peripheral region (Figure 5),
suggesting that AtPP2C52 interacted with UMP1 in
the plasma membrane. AtPP2C52 interacted with
RD21a not only in the plasma membrane but also in
the nucleus (Figure 5). These results suggest that
RD21a and UMP1 are the potential substrates of
AtPP2C52.

