基本HTML版本

Computational Molecular Biology 2015, Vol. 5, No. 1, 1-13
http://cmb.biopublisher.ca
11
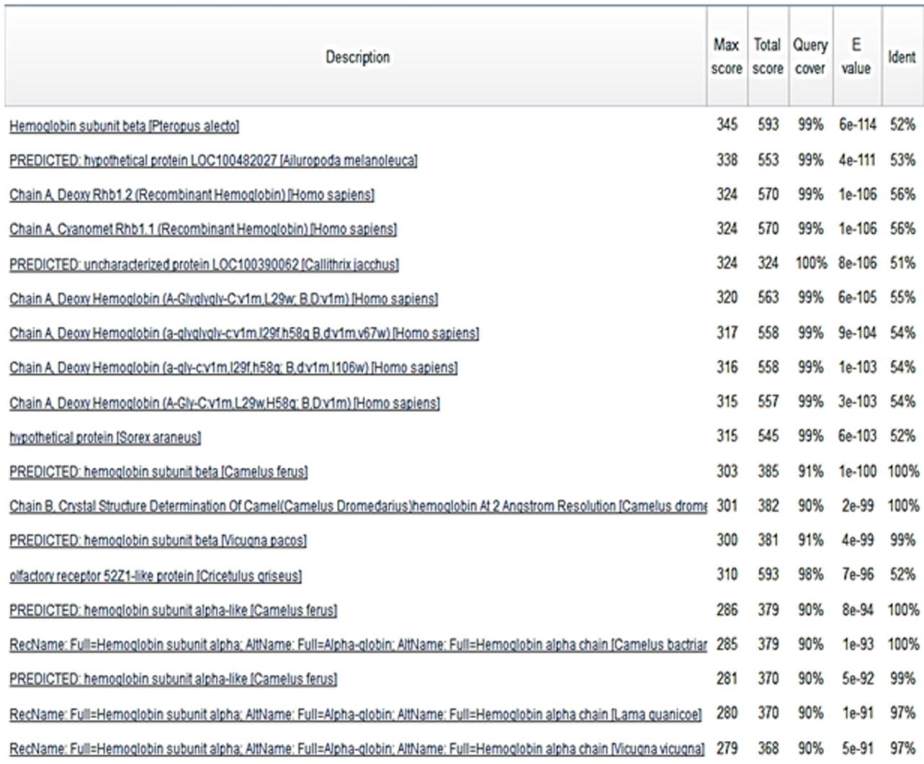
The result of PSI-BLAST (Position-Specific
Iterated BLAST) for α and ß chains hemoglobin of
domestic camels (HBA and HBB) are shown in
Figure 4. We found a lot of HomoloGene between
camel HB versus other species such as
Pteropus
alecto
,
Ailuropoda melanoleuca
,
Callithrix jacchus
and etc (Figure 4). Anyway it was interesting when
we found hemoglobin in domestic camel has a lot of
HomoloGene versus different mammalian even
Pteropus alecto
or bat.
Figure 4 PSI-BLAST (Position-Specific Iterated BLAST) between α-chain (HBA) and ß-chain (HBB)
Camelus ferus
,
dromedarius
and
bactrianus
with different species from GenBank. We found a lot of HomoloGene from different species that each them have
identity with α-chain and ß-chain hemoglobin of
Camelus ferus
,
dromedarius
and
bactrianus
(HBA). Maximum score, total score,
query cover, E-value and ident are shown as genome homology
3 Conclusions
The findings of the current study led us to conclude
that camel HBB appeared to be no susceptible to
N-glycosylation than human. Based on isoelectric
point, camel HBB and HBA has high mobility, and
thus affects to glycosylation. We know alpha and beta
subunits of hemoglobin have a lot functions such as
biological process, molecular function and
involvement in disease but most importantly, involved
in oxygen transport from the lung to the peripheral
tissues. Despite, the heme is common ligand of HBA
and HBB in all species; it can link to the oxygen by
ferrous. As well as, O-linked glycosylation occurs
than N, C-linked glycosylation; however camel HBB
is resistant to O-linked glycosylation. Although, alpha
chains of hemoglobin is more susceptible to

