基本HTML版本

Computational Molecular Biology 2015, Vol. 5, No. 1, 1-13
http://cmb.biopublisher.ca
8
2.5 Protein structure modeling
The prediction of tertiary structure for alpha and beta
chains (HBA and HBB) based on homology-
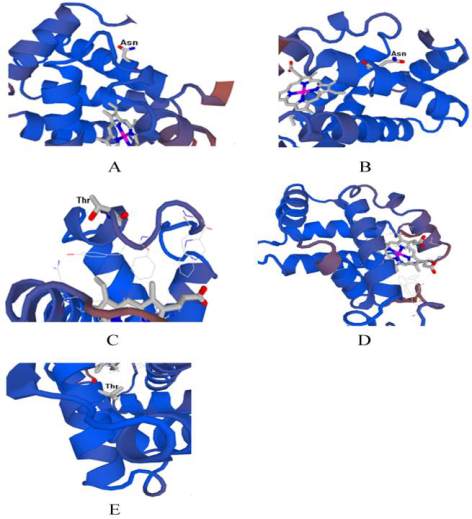
modelling using the ExPASy web server has shown in
Figure 3. Tertiary structure of HBA was quite similar
between
Camelus
family and N-linked glycosylation
was Asn that illustrated in Figure 3. It might be caused
by each mutations happened into α-chain of Hb. So
through our findings, most critical functions of
hemoglobin for instance oxygen transportation doesn’t
been accomplished by other kinds of subunits such as
gamma, delta and zeta. In fact α and ß-chains probably
controlled most critical functions of hemoglobin. As
showed in Figure 3 tertiary structure of HBB and
HBA were all the same in
Camelus
family but it was
different than
Homo sapiens
(Figure 3). As on Figure
4, the ligands of HBA and HBB in the mentioned
species were heme. Role of heme is linked to oxygen,
both hemoglobin subunits affected to oxygen transport.
We have seen in this article that the based on
homology modeling, tertiary structure of HBB in
Camelus
family are much likely to α-chain of the
Homo sapiens
(Figure 3). Despite the fact that, the
based on Figure 3, HBB in
Camelus
family have not
susceptibility position to glycosylation. As seen in
Figure 3, the Asn131 and Asn132 are susceptible
amino acids to N-glycosylation in HBA of domestic
and wild camels respectively however all Asn in
human HBA are resistance to N-glycosylation.
According to similar structure between human HBA
and camel HBB, we conclude that the resistivity
against N-glycosylation is done by HBA in human.
Whereas we demonstrated as Figure 3, HBB has less
position for N-glycosylation than HBA; we conclude
HBB is more involved in critical actions than HBA.
As previously mentioned sequence of HBB is highly
conserved in all considered species, perhaps this
explains the crucial role of hemoglobin in oxygen
transports.
2.6 Glycosylation sites
The prediction of glycation which is a non-enzymatic
binding of glucose to the protein (as in the case of
HbA1c) was done based on binary profile of patterns
(BPP). The result of glycosylation sites for α and ß
chains is shown in the Table 4. In this research, no
Figure 3 The tertiary structure of predicted model for the whole
sequences. 3A) HBA Camelus ferus. 3B) HBA
Camelus
bactrianus
and
dromedarius
. 3C) HBA
Homo
sapiens
. 3D)
HBB
Camelus ferus
,
Camelus bactrianus
and
dromedarius
. 3E)
HBB
Homo sapiens
. The predicted 3D model(s) for every
domain are shown, that each chains of hemoglobin only have
one domain (domain 1) in the mentioned species. As shown in
Fig. 3 A and B, Asn is pottential N-glycosylated in camel but
Thr is pottential O-glycosylated in Fig. 3 C, D and E. The
ligand of hemoglobin (Heme) is illustrated too
C-linked glycosylation is found for any HBA and
HBB in mentioned species. The C-linked
glycosylation is comparatively rare event and in this
the glycan is found attached to carbon of the first Trp
residue in the consensus sequence W-X-X-W or
W-X-X-C or W-X-X-F (where X is any AAs) (Krieg
et al., 1998). Based on Table 4 for N-glycosylation in
HBA we found a potential glycosylated site for
Asn131 in
Camelus bactrianus
and
dromedarius
showed and Asn132 for
Camelus ferus
but
Homo
sapiens
show no potential N-glycosylated site for both
hemoglobin subunits (Table 4). N-linked glycosylation
is recognized by addition of glucose to a nitrogen
atom, usually the N4 of Asn that specifically
recognizes a consensus sequence Asn-X-Ser/Thr,
where X is any amino acid except prolin (Gavel and

