Basic HTML Version



Molecular Plant Breeding 2011, Vol.2, No.15, 101
-
108
http://mpb.sophiapublisher.com
103
Table 1 Comparison of compositions and physical and chemical characteristics of nucleotide sequences and deduced amino acid
sequences of RuBisCo large subunits among different higher plants
Item
Rice
maize
Arabidopsis
Pea
Orange
Moth orchid
Length of ORF (bp)
1434
1431
1440
1428
1428
1464
Initiation codon
ATG
ATG
ATG
ATG
ATG
ATG
Termination codon
TAG
TAA
TAG
TAA
TAA
TAA
Number of deduced AA
477
476
479
475
475
487
Molecular weight (kDa)
52.88
52.70
52.95
52.76
52.52
54.04
Theoretical isoelectric piont (pI)
6.22
6.33
5.87
6.55
6.29
5.96
Gly (9.6%)
Gly (9.9%)
Gly (9.8%)
Gly (9.7%)
Ala (9.9%)
Gly (9.7%)
Ala (9.4%)
Ala (9.5%)
Ala (9.0%)
Ala (9.3%)
Gly (9.7%)
Ala (9.0%)
Leu (7.8%)
Leu (7.8%)
Leu (8.6%)
Leu (8.6%)
Leu (8.6%)
Leu (7.8%)
Glu (6.9%)
Glu (6.5%)
Glu (7.3%)
Val (6.7%)
Val (6.9%)
Glu (7.0%)
The most abundant AA
Val (6.5%)
Thr (6.5%)
Val (6.9%)
Glu (6.5%)
Glu (6.5%)
Val (7.0%)
Acidic AA (%)
12.58%
12.40%
12.73%
12.21%
12.21%
13.14%
Alkaline AA (%)
11.32%
11.34%
10.65%
11.58%
11.16%
11.29%
Total electric AA (%)
23.90%
23.74%
23.38%
23.79%
23.37%
24.44%
Polar AA (%)
21.38
21.43%
21.92%
21.47%
21.47%
21.15%
Hydrophobic AA (%)
34.80%
34.87%
35.07%
35.37%
36.00%
34.50%
Instability index (%)
43.50%,
unstable
42.57%,
unstable
42.31%,
unstable
39.90%,
stable
37.47%,
stable
42.30%,
unstable
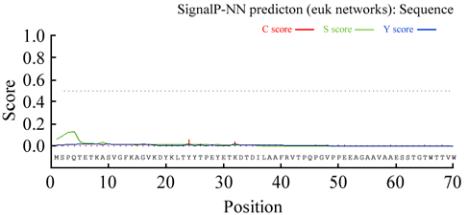
Figure 1 Signal peptide prediction of rice rbcL
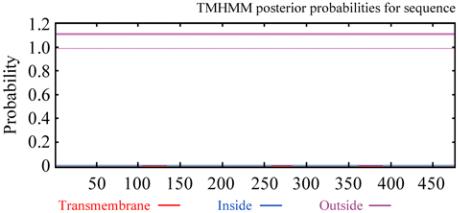
Figure 2 Transmembrane topological structure analysis of rice
rbcL
1.4 The hydrophobicity and hydrophilicity analysis
of plant rbcLs
The hydrophobicity and hydrophilicity analysis of the
rbcL AA sequence of
Oryza sativa subsp. japonica
was fulfilled with ProtScale program (Kyce and
Doolittle, 1982). The most hydrophilic AA residue in
the polypeptide is Asn, located at 306
th
, because of the
lowest score of
-
2.644. And the most hydrophobic AA
residue is Ala, situated at 378
th
, which has the top
score of 1.778. As for the whole polypeptide, the
hydrophobic and hydrophilic AA residues distribute
uniformly, but the number of hydrophilic AA residues
is higher than that of hydrophobic AA residues, and
any obvious hydrophobic AA residues concentrative
region can't be detected (Figure 3). Similar
distributive rule of hydrophobic and hydrophilic AA
residues was found in other rbcL AA sequences from
Nicotiana tabacum, Lolium perenne, Medicago
truncatula, Pisum sativum,
and
Citrus sinensis
. Thus,
the results implies that the rbcLs in higher plants are
hydrophilic protein, which is in accord with the
previous conclusion that transmembrane topological
structure is absent in rbcLs of higher plants.

