Bt Research 2015, Vol.6, No.3, 1-10
5
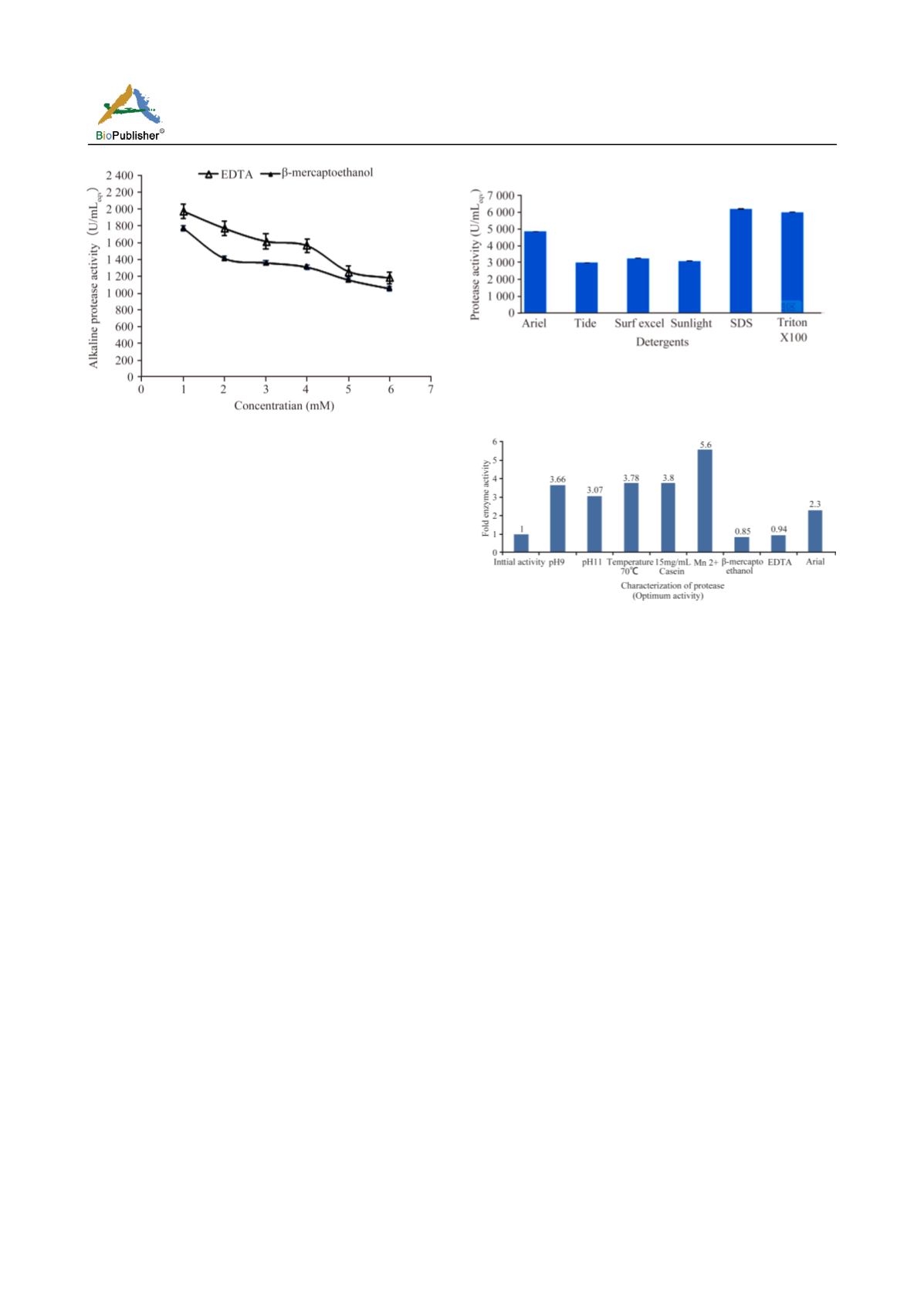
Figure 5A. Jisha et al. (Color in web edition only)
5A. Effect of EDTA and
β
- mercaptoethanol on protease
activity.
U/ml
eqv
) and 15% (1741 U/ml
eqv
), respectively; in
comparison to initial activity (Figure 5A).
At the optimized reaction conditions, the presence of
detergents in the reaction system variously affected the
protease activity. Protease activity was not disturbed
when incubated with different concentrations (0.1, 0.2,
0.4, 0.8 or 1%) of surfactants like SDS and Triton
X-100. Presence of SDS or Triton X-100 slightly
affected the protease activity;
i.e
., in the presence of
0.2% SDS or Triton X-100, 12% reduction in activity
was noticed, which was in comparison to the activity
at optimized conditions (Figure 5B).
Unlike SDS, and Triton X-100, protease showed good
stability in the presence of commercial detergents
tested (Ariel, Tide, Surf Excel or Sunlight);
i.e
., the
maximum stability showed in the presence of Ariel
with activity 4867 U/ml
eqv
and was over 2.3 folds
increase over the initial activity (Figure 5B); in other
words 40% of the maximum activity obtained at the
optimized conditions. The fold increase is summarized
in Figure 6.
Discussion
Employing biphasic fermentation strategy, our group
already demonstrated the amylase produced by
Btk
as
a by-product during the process of the production of
δ
-endotoxin (Smitha et al. 2013a; Smitha et al. 2013b;
Jisha et al. 2014; Smitha et al. 2015b). Based upon
this biphasic fermentation strategy, a detergent- and
thermo-tolerant protease is demonstrated in the present
study.
Figure 5B. Jisha et al. (Color in web edition only)
5B. Effect of commercial detergents and surfactants on protease
stability on alkaline protease stability.
Figure 6 Jisha et al. (Color in web edition only)
Consolidated data showing the maximum protease activity
under optimized reaction conditions
Characterization of enzyme is an important step
toward developing a better understanding on the
functioning of the enzyme (Yadav et al., 2010). Alkaline
protease in the culture supernatant could be purified
by the conventional procedures involving fractionation by
ammonium sulphate, molecular weight cut-off membrane
filtration, and molecular sieving using sephadex G-100
molecular sieving. Precipitation by ammonium sulphate
is the conventionally used method for the purification
of protein from the crude extract (Bell et al., 1983). In
the present study, the specific activity of the protease
increased from 138 U/mg (crude protease) to 1766
U/mg, after final step of purification (sephadex G-100),
i.e
., 12.79 folds purification and 0.3% overall yield.
These results indicate the effectiveness of purification
method. Various percentage yields and purification
folds were reported for proteases from various species
of
Bacillus:
Bacillus
sp. K25 with 40% yield and
10.08 folds purification (Mathew 1999) ;
Bacillus
sp
.
PS719 with 39% yield and 18.5 folds purification
(Hutadilok-Towatana et al., 1999);
B. subtilis
with
7.5% yield and 21 folds purification (Adinarayana et
al., 2003); and
Bacillus
strain HS08 with 5.1% yield


