Basic HTML Version



Plant Gene and Trait, 2013, Vol.4, No.6, 30
-
36
http://pgt.sophiapublisher.com
31
promising feedstock for bioethanol production, large
scale commercial production plants are not in use yet
in the US or EU because of the high production costs
(Sticklen, 2008). Procession lignocellulose into
bioethanol occurs in four main steps: pretreatment,
hydrolysis, fermentation and product separation.
Pretreatment with chemical (acid), biological or
mechanical method is the first step in order to reduce
the size of the lignocellulosic material, hence
increasing the hydrolysis rate of cellulose and
hemicellulose in the second step (Mosier et al., 2005).
Hydrolysis is to degrade the macromolecular cellulose
and hemicellulose into monosaccharides, including
hexose, xylose, mannose, galactose, arabinose and
other oligosaccharides. Monosaccharide will be
fermented into ethanol under the action of the
enzymes. The last step involves a distillation and high
purity ethanol will be achieved (Arshadi and Sellstedt,
2008). At present, among the industry chain from
lignocellulosic biomass into bio-ethanol, a major
limitation is the recalcitrance of lignin, which
decreased the saccharification efficiency. Because the
hydrolysis enzymes are expensive, thereby greatly
increasing the cost of production of bio-ethanol
(Chen et al., 2002).
Lignin, one kind of phenylpropanoid derivatives
deposited on the cell wall of vascular plants, is
composed of polymer compound with high molecule
weight up to hundreds to millions connected by three
lignin monomers by ether bond or a carbon-carbon
bond, which accounts for 15%~35% weights of plants
(Zhong et al., 2000). Lignin can enhance the
mechanical strength of plants, improve cell transport
capacity (Whetten and Sederoff, 1995), and can resist
against pathogenic microorganisms (Duthie and
Crozier, 2000). Lignin biosynthesis of vascular plants
is an important evolutionary adaptation characteristic
to terrestrial environment. Biosynthesis of lignin
monomer is a very complex physiological and
biochemical process in plant, which is the result of
phenylpropanoid pathway conducted by deamination
of phenylalanine (or tyrosine) into cinnamic acid,
through a series of hydroxylation, methylation and
reduction reactions, which ultimately produce three
kinds of main monomer, namely syringyl lignin
(S-lignin), guaiacyl lignin (G-lignin), p-hydroxyphenyl
lignin (H-lignin) (Fan et al., 2005).
Cinnamate-4-hydroxylase (C4H) and cinnamoyl
alcohol dehydrogenase (CAD) are two key enzymes of
lignin biosynthesis (Vanholme et al., 2010; Hamada et
al., 2004). In this experiment, the total RNA was
extracted from Miscanthus with CTAB-LiCl method.
Based on the cDNA sequences of
C4H
and
CAD
isolated
from several monocots reported in Genbank, two pairs
of PCR primers were designed. Cloning and
bioinformatic analysis of cDNA fragments of
C4H
and
CAD
genes were carried out, hence laying a
foundation for the next full-length cloning
of
C4H
and
CAD
genes with RACE (rapid amplifi-
cation of cDNA ends) technique and gene expression
analysis with qPCR (Real-time Quantitative PCR)
technique in the future.
1 Results and analysis
1.1 Extraction of total RNA
After the total RNA was extracted from
Miscanthus
with
CTAB-LiCl method and then was digested with
DNase I, the purified RNA was detected with the
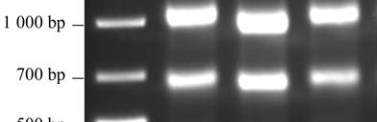
agarose gel electrophoresis (Figure 1). It indicated that
two bands of 28S and 18S rRNA were clear enough,
and the brightness of the 28S rRNA band to 18S rRNA
is about 1.5 times, which showed that the extracted

