Basic HTML Version

Molecular Plant Breeding 2013, Vol.4, No. 30, 247
-
253
http://mpb.sophiapublisher.com
251
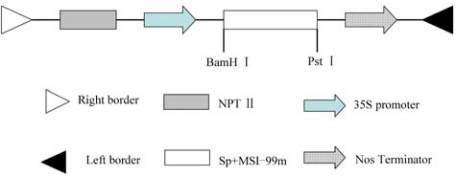
Figure 6 Structure of recombined pGS-
MSI99
vector
Note: pGS construct containing the
MSI-99m
gene fused to the
signal peptide (SP) and under the control of the CaMV 35s;
The components in the figure are as follows: RB and LB, the
right and left border regions of the Ti plasmid; 35s promoter
and NOS terminator, respectively; NPT II, neomycin
phosphotransferase II; SP+MSI-99m, the sequence of signal
peptide and
MSI-99m
structure of expression vector pGS-
MSI-99m
0.2 in MS medium supplemented 200 μM
acetosyringone. Cotyledons from 6-day-old seedlings
were excised with 2 mm petiole at the base and
hypocotyls were cut to 0.5 cm segments. The explants
were immersed in the bacterial suspension for 5 min.
They were subsequently placed on RM
1
(solid MS
medium in 500 mg/L MES buffer supplemented with
1.0 mg/L 2,4-D and 200 μM acetosyringone, pH 5.2)
for co-culture at 25°C for 2 days in the dark. After
co-cultivation, the explants were washed with sterile
water containing 300 mg/L cefotaxime (Cef) to inhibit
the growth of
A. tumefaciens
on the explants surface
transferred to RM
2
(solid MS medium with 3.5 mg/L
6-BA, 0.1 mg/L NAA, 5.0 mg/L AgNO
3
and 300 mg/L
Cef) for 2 weeks. After that, the explants with callus
were transferred to RM
3
(solid MS medium with
3.5 mg/L 6-BA, 0.1 mg/L NAA, 5.0 mg/L AgNO
3
,
300 mg/L Cef, and 10 mg/L Kan) for shoot induction.
Subculture of the explants was done on fresh RM
3
medium every 2 weeks. Green shoots (approximately
3 cm in length) were excised from the explants and
transferred to RM
4
(solid MS medium with 0.2 mg/L
NAA, 300 mg/L Cef, and 15 mg/L Kan) for rooting
and recovering of complete plants. Except for the RM
1
medium, all of the other aforementioned media were
adjusted to pH5.8. Cultures were maintained at 25
℃
with the photoperiod of 16 h/8 h (light/dark). One
month later, surviving green plantlets on medium with
Kan were transplanted in pots in a greenhouse for
molecular identification and evaluation of their
disease resistance.
3.4 PCR analysis
The presence of the
MSI-99m
gene in transgenic
plants was detected by PCR. The fresh leaves of
transformed and control plants were used to extract
genomic DNA following a modified SDS method
(Hu
et al., 2003)
. The transgenic plants were identified
with the primer pair (Primer 1: 5'ATTGATGTGATAT
CTCCACTGACGTAAG and Primer 2: 5'TCTGCAG
TTAAGAATTAAGAATTTCCTT). The PCR product
was expected to be a 400-bp fragment containing the
partial sequence of the pGS vector. PCR amplifications
were performed with the following thermal cycling
conditions: 94
℃
for 4 min, 30 cycles of 94
℃
for 1 min,
57
℃
for 40 s, 72
℃
for 35 s; followed at 72°C for 10
min. The purified product was then cloned into the
pMD-19T vector (TaKaRa) for sequencing (Beijing
Genomics Institute, China).
3.5 Quantitative Real-Time PCR Analysis
To detect the expression of the
MSI-99m
gene in
transgenic plants, Total RNA was isolated from the
leaves of 4-week-old transgenic and control plants
using Trizol reagent (Invitrogen, USA) according to
the manufacturer’s instructions and treated with
RNase-free DNase to remove genomic DNA. The
cDNA was synthesized using the TaKaRa Reverse
Transcription System kit (TaKaRa Biotech, Dalian,
China). The rape
EF-1α
gene was used as an internal
control. The amplification was performed with Primer 3:
(5'ATGCTTCTTGCTATTGCTTTTCTTGC) and Primer
4: (5'TCTGCAGTTAAGAATTAAGAATTTCCTT)
designed according to the
MSI-99m
sequence. The
qRT-PCR was carried out on an Applied Biosystems
7300 Real Time PCR System with a 20 µL reaction
volume, containing 1µL 10-fold diluted cDNA, 0.3 µL
(10 pm) of each primer , 10 µL SYBR
®
Premix Ex
Taq
TM
(Perfect Real Time) (TaKaRa Code:DRR041A)
and 8.4µl sterile double distilled water. The PCR
conditions consisted of denaturation at 95
℃
for 4 min,
followed by 40 cycles of 95
℃
for 20s, 57
℃
for 20s
and 72
℃
for 40s. The specificity of the individual
PCR amplification was checked using a heat
dissociation curve from 55 to 95
℃
following the final
cycle of the PCR. The correspondence expression
content (2−ΔΔCT) of
MSI-99m
mRNA was calculated
as following: ΔΔCT=(C
T.Target
− C
T.
EF-1α
)×X−(C
T.Target
–

