Basic HTML Version




Bioscience Methods
BM 2011, Vol.2, No.5
http://bm.sophiapublisher.com
- 35 -
efficiency of
GUS
gene by 15 minutes of infection is
31.43%, 30 minutes’ infection is 47.14% and 45
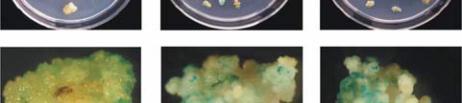
minutes’ infection is 27.14% (Table 6). As shown in
Figure 4, the callus looked to be darker by GUS
staining after 30 and 45 minutes of infection than that
of 15 min of infection. But when the callus was
infected by
Agrobacterium
containing
GUS
gene 45
minutes, the transient expression efficiency was low
(Table 6). These results indicate that 30 minutes of
infection was appropriate to the transformation of
recombinated Agrobacterium (OD
600
≈0.8~1.0).
Table 6 Result of
Puccinellia haupitiana
embryo callus transf-
ormation efficiency at different dipping time
Infection
time (min)
Differentiation
rate (%)
Browning
rate (%)
15
12.56±1.84
80.34±1.66
30
25.42±1.76
70.23±1.42
45
42.35±1.68
50.45±1.59
Note: NT refers to the total number of callus involved in the
infection test; NB refers to the number of callus turning to be
blue by GUS staining that meant the transient expression of the
GUS
gene
Figure 4 GUS staining of callus with different infection time by
recombinated
Agrobacterium
containing
GUS
gene
2 Discussion
In summary, 2,4
-
D is a necessary regulator in the
process of callus induction of gramneal plants
(Mitsuoka et al., 1994). In this study, the highest
induction rate we have got is 39.75% using the altered
medium I supplemented with 4 mg/L 2,4
-
D as the
induction medium (Table 1 and Figure 1). Among the
grass family, the callus induction of
Puccinellia
chinampoensis
needs more 2,4
-
D than others. Besides
this regulator, the composed elements, such as C
source (Lee et al., 2002), N source (Grimes and
Hodges, 1990), amino acids (Ozawa and Komamine,
1989) (Chowdhry et al., 1993) and iron salts, can also
affect the callus induction. Adjustment the contents of
these elements can change the induction rate, as
shown in Table 2 and Figure 2.
In the process of callus subculture, adding a certain
amount of ABA can effectively improve the quality of
callus (Higuehi and Maeda, 1990). In the callus
induction and subculture process, the rational use of
nitrate and ammonium nitrogen will be more
beneficial to the formation of embryogenic callus (Ge
et al., 2006), which was determined in this study
where the modified medium S was chosen to be the
best medium for the callus subculture (Table 3).
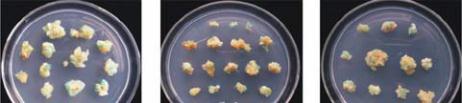
About the callus differentiation, proembryogenic
masses (PEMs) in the embryogenic callus can
gradually develop into somatic embryos at appropriate
culture conditions, and further differentiate into a
complete plant (Arnlod et al., 2002). However,
non-embryogenic callus is composed by relatively
bigger cells, which have large vacuoles but little
cytoplasm, and there are less PEMs in the surface of
callus. High proportion of cytokinin / auxin is more
conducive to callus shoot differentiation, but if the
hormone levels are too high, it will reduce the
differentiation rate and increase the callus browning
(Laukkanen et al., 1999), as shown in Table 5. A
certain amount of proline added into the induction,
subculture and differentiation media can suppress the
callus browning (Tang and Newton, 2004).
In the callus genetic transformation test by
Agrobacterium
,
the infection time is one of the primary factors that
affect the transformation rate. Long time of infection
will inhibit the growth of the receptor, hard to get the
tranformants, but short time of infection go against the
attachment of
Agrobacterium
to the receptor and
decrease the transformation rate (Peng et al., 2005). To
the callus transformation of
Puccinellia chinampoensis

