Basic HTML Version


45q3
International Journal of Molecular Veterinary Research
2012, Vol.2, No.1, 1
-
5
http://ijmvr.sophiapublisher.com
2
1 Results
Regression analysis was performed on the optical
density (OD) data of sera, the presence of antigens of
BVDV in sera of group (A) were
in all samples. The
OD of 45 samples were above 0.39 whereas the OD of
five samples were from 0.2 to 0.39 and these samples
were retested and the OD values were above 0.2 so all
A group samples considered as positive, whereas the
OD of B group samples were under 0.2 and these
samples considered as negative.
The slope of standard curve shows 100% efficiency,
when the concentrations of primers (10 pmol/µL) and
1\1 cDNA were used.
About sensitivity of the PCR the assay could detect;
10 to 100 TCID50 / ml in samples.
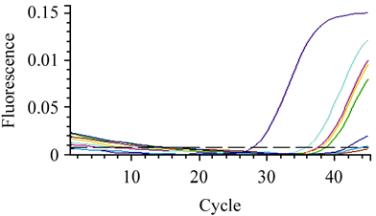
Threshold cycles (CT) values of the positive control
on SYBR Green assay was 28 while the threshold
cycle (CT) of A group were under 35 for 45 samples
which considered positive samples and from 35 to 37
for five samples which considered weak positive
samples, whereas the CT values of B group were
above 40 and considered as negative (Figure 1). The
melting curves of the positive controls and positive
samples were the same (85
℃
).
Figure 1 Results of the real-time PCR
Note: Purple line: Positive control sample; Blue: Positive test
samples; Pink, Yellow and green weak positive test samples;
Other lines: Negative control and negative samples
2 Discussion
2.1 Antigen-capture ELISA
Culling of the persistent infected animals is essential
to control BVDV infection in the herds. Therefore, it
is important to perform a reliable, rapid and specific
test to detect BVDV. For this, numerous tests have
been used such as ELISA, RT-PCR, real-time RT-PCR
and immune-histochemistry as well as virus isolation
(Saliki et al., 2004).
Several methods for antigen detection by ELISA have
been published (Vanderheijden et al., 1993; Cornish et
al., 2005; Entrican et al., 1995) and several
commercial kits are available. Most are based on the
sandwich ELISA principle, with a capture antibody
bound to the solid phase, and a detector antibody
conjugated to a signal system such as peroxides. The
new generation of antigen-capture ELISAs (ERNS
capture ELISAs) is able to detect BVD antigen in
blood as well as in plasma or serum samples.
These assays for the detection of viral antigens (Ag)
has made testing fast and somehow cheaper, according
to the manufacturer the kit has specificity>99.7 and
sensitivity approaching 100% in tested populations,
our results indicate to 100% specificity and sensitivity,
the results of the current study agree with those of
Kampa et al (2007).
2.2 Polymerase chain reaction PCR
A reverse transcriptase-polymerase chain reaction
RT-PCR technique has previously been described for
the detection of BVDV in tissues (Belak et al., 1991),
in cell cultures (Hertig et al., 1991; Baxi et al., 2006)
and frozen blood samples (young et al., 2006). A
combined RT-PCR has been described for its detection
in whole blood and tissues (Hamel et al., 1995) and
has been applied to the detection of persistent
infection (PI) animals in milking herds, through
examination of somatic cells from bulk milk (Radwan
et al., 1995).
Real-time PCR has many advantageous because of its
sensitivity, specificity, rapidness and testing many
samples by pooling. However, one must be careful
when performing not to get false positives due to
contamination.
Baxi and others (2006) have reported that result of
virus isolation and real-time PCR were agreed in 100
samples tested. Hilbe and others (2007) have also
compared five diagnostic tests and they found that
three antigen detection methods (including ELISA)

