Molecular Plant Breeding 2015, Vol.6, No.14, 1
-
8
4
e
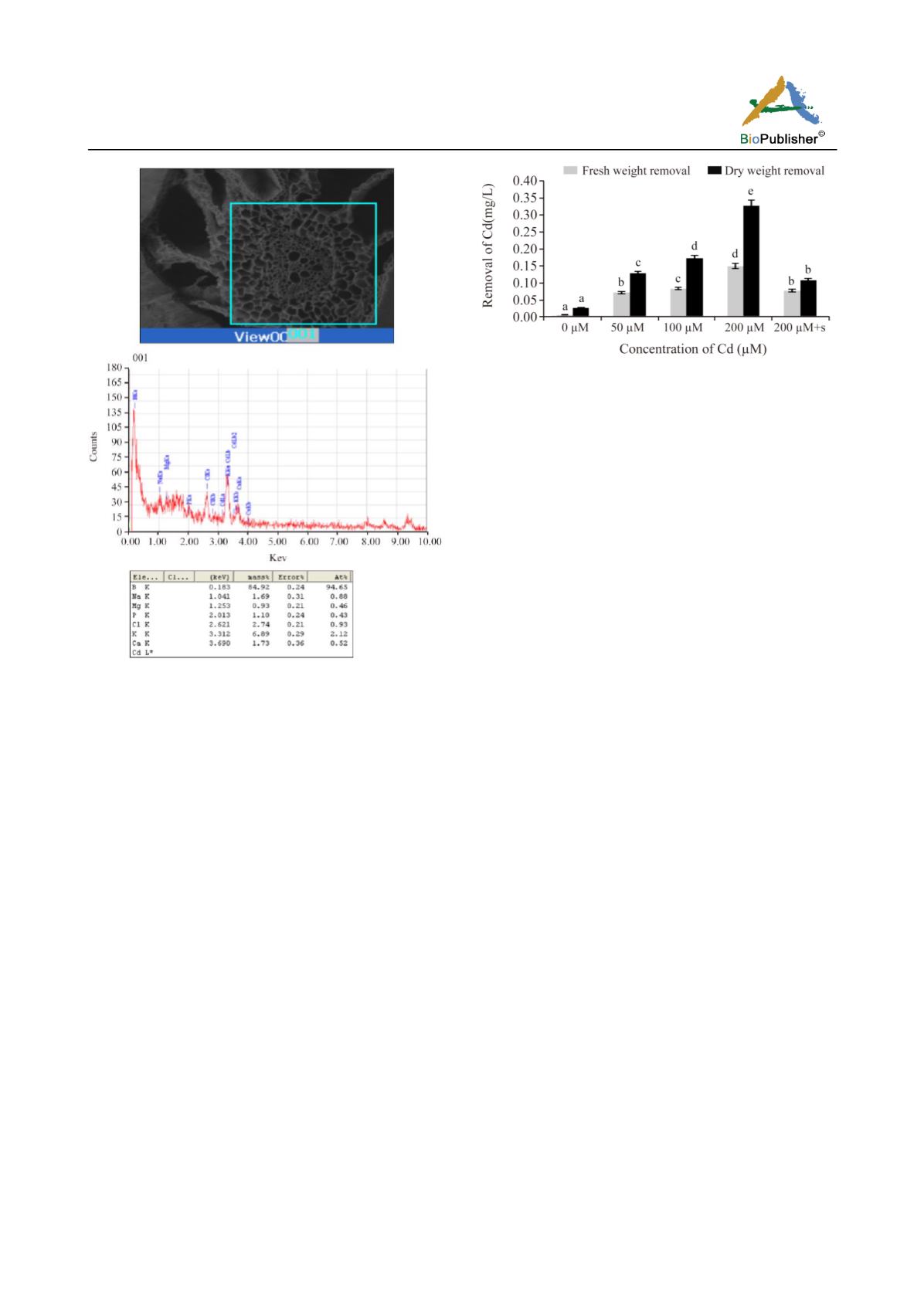
Figure 1 a-1e EDAX spectra of
Marsilea
plant of a) 0 µM Cd,
b) 50 µM Cd, c) 100 µM Cd , d) 200 µM Cd and e) 200 µM
Cd+2 mM spermidine
In a mixture with more than one substance,
particularly, with the biomolecules, there are some
physical bending of functional groups against
chemical interactions. This is more supported with
changes in bio-adsorption pattern with varying states
of biomass. In the present experiment also
Marsilea
plant had followed the same pattern. The dry and fresh
biomass showed a significant variation in metal
adsorption in a range of 5.15, 6.98, 13.35 fold and
1.97, 2.31, 4.2 fold respectively over control. With the
application of spermidine (Spd), the plants were less
absorbed by 67.48% and 48.97% in dry and fresh
biomass respectively when compared to 200 µM Cd
(Figure 2). This is interesting to note the changes in
characteristic features of bands in FTIR analysis for
different bio-molecules as cell wall constituting
residues (Figure 3). In the present experiment, the
spectra from varying Cd concentrations had shown
appreciable variations. However, the peak maximum
of each frequency was less altered but with some
changes in the shapes as well as intensities also. FTIR
Figure 2 Adsorption of metals by
Marsilea
plant in fresh and
dry sample states under different Cd concentrations
analysis provides the spectra of each such mixing
moiety with some characteristic peaks for chemical
modifications. Likewise, the chemical changes of cell
surfaces of
Marsilea
under Cd stress supplemented
with Spd were featured by FTIR spectroscopy
(Lesmana et al., 2009). The physicochemical analysis
of this process suggested that bio-adsorption is
facilitated by complication, coordination, ion-exchange,
chelation and adsorption of metals. With reference to
plants, the removal of metals is facilitated with an
initial reversible bio-adsorption and secondarily, a
slow but steady irreversible bio-accumulation. The
latter is also referred to as ion sequestration when
specific sub-cellular fractions are engaged in the
tissues. This was more confirmed with FTIR studies
that had shown in the figures (Figure 3). For the
comparative analysis of samples from varying Cd
concentrations and with Spd treatment, it recorded a
distinct variable trend suggesting the impact of heavy
metals on
Marsilea
and its responses on cell wall
moieties. This was also confirmed and concluded from
many experiments for the efficacy of plant biomass
for bio-adsorption of metals. This was also
documented from our
Marsilea
plant irrespective of
dry and fresh state of biomass. From the tables of
changes in the frequency regions of different
functional groups of biomolecules on the cell wall are
expected to be involved for heavy metal (e.g. Cd in
the present case) adsorption (Table 1). This probably
is indicative of the facts of heavy metal induced
bio-adsorption with some functional groups with
changes in bond strength. From the Table 1, the major
affected functional groups are detected as alkyl halide
(-C-Cl) with frequency region 770-772 cm
-1
, the
amino functional group is more dissected with C=O
(with frequency region 1652 cm
-1
and N-H (with
frequency region 3409-3399 cm
-1
) (Esteves et al.,
2013). Moreover, in earlier studies, it reported that any


