Basic HTML Version

Molecular Entomology 2013, Vol.4, No.3, 13-21
http://me.sophiapublisher.com
14
Many plants have received gene/s that encoding toxic
proteins as a strategy to resist or be protected from
insect pests and pathogens. Genes that their products
are toxic to insect species and confer enhance
resistance against pest that so far have been received
great attention are lectins (Gatehouse et al., 1997),
α-amylase inhibitors (Mehrabadi et al., 2012;
Mehrabadi and Bandani, 2011), protease inhibitors
(Saadatia et al., 2011), toxins from
Bacillus
thuriengiensis
(Bt toxins) (Sharma and Ortiz, 2000),
and even fusion proteins consisting of plant lectin,
Galanthus nivalis
agglutinin (GNA) linked to toxic
peptide (Fitches et al., 2004; Down et al., 2006;
Fitches et al., 2009).
Digestive system of insects is a good target for
implementing control mechanisms that are not toxic to
other organisms (Nauen et al., 2001). A large number
of proteinaceous and non-proteinaceous molecules in
plants act as part of the plant's natural defense against
herbivores in order to prevent herbivors feeding
(Mendiola-Olaya et al., 2000; Franco et al., 2002;
Piasecka-Kwiatkowska et al, 2007). These inhibitors
are widely existed in various plant seeds and tubers
with greater abundance, especially in cereals and
legumes (Iulek et al, 2000; Svensson et al, 2004;
Bonavides et al, 2007; Mehrabadi et al., 2010). Thus,
one important aspect of the insect pest control is to
materialize selective inhibition of the digestive
enzymes thus to interfere in digestion process of the
insect by producing detrimental effects on larval and
insect growth by inhibition of the digestion and
assimilation of nutrients. Therefore, in order to
achieve a control strategy based on digestive enzyme
inhibitors, it is advisable to characterize digestive
enzymes as well as to do
in vitro
and
in vivo
bioassays
with plant proteinaceous inhibitors (Horrison and
Bonning, 2010).
A first example of introduction of plant genes
encoding toxic protein against insect is cowpea trypsin
inhibitor which expressed in tobacco leaves to combat
lepidopteran larvae (Hilder et al., 1987, Silva et al.,
2001). Since then, attempts have been made to use
toxic plant proteins against insects with some success.
α-Amylase inhibitor gene from seeds of common bean
(
Phaseolus vulgaris
) when transferred to pea confer
resistance to pea weevil (
Bruchus pisorum
) (Morton et
al., 2000; Silva et al., 2001). Also, transformed Azuki
bean (
Vigna angularis
) confers resistance to
Callosobruchus chinensis
and
C. Maculates
(Ishimoto
et al., 1996; Silva et al., 2001).
So far, no investigation has been done on the effect of
plant origin toxic metabolites on the carob moth gut
digestive enzymes. Thus, the aim of the current study
was to investigate the effect of cereal seed
proteinaceous extract including wheat cultivars and
triticale against the insect gut digestive enzymes using
spectrophotometric and in gel assays procedures.
2 Results
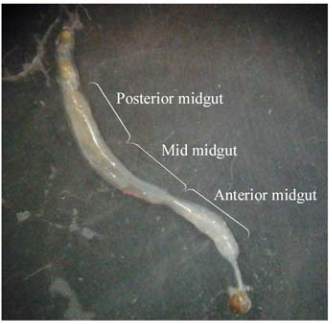
2.1 Determination of enzymes activity in different
parts of the midgut
The α-amylase enzyme activity in the anterior, mid
and posterior was 0.013 U/min/mg protein, 0.018
U/min/mg protein and 0.016 U/min/mg protein,
respectively (Figure 1 and Figure 2A). Fgiure 2B
shows α-amylase activity in the gel assay that two
α-amylase bands are seen in the insect gut, one is A1
which is the major α-amylase band and the other is A2
with minor activity.
Figure 1 Different part of the carob moth midgut
The proteolytic activity of the three gut parts including
anterior, mid and posterior gut was 0.003 U/min,
0.004 U/min and 0.003 U/min, respectively (Figure
3A). As shown in figure 3B, there are two protease
bands in the anterior, mid and posterior part of the
midgut. However, Bands of mid part were sharper
than the anterior and posterior midgut showing higher
activity of these proteases in the middle part of the
midgut (Figure 3B). These data showed that in the
both enzymes (α-amylase and protease), activities
were more in the midsection of the midgut than those
of the posterior and anterior midgut.

