Basic HTML Version

International Journal of Marine Science 2015, Vol.5, No.9, 1-4
http://ijms.biopublisher.ca
2
divided equally and immediately preserved in four
bottles containing different fixatives; 70% Alcohol, 5%
formaldehyde and glutardialdehyde in the proportion
3:2, 1% Lugol’s solution and filtered seawater
(control), respectively. After 10 minutes of exposure
of mussels to the fixatives, all the four bottles were
stored in ice.
1.2 Phytoplankton identification and quantification
In laboratory (about 4 hours later), the valves were
separated apart and the stomach content of specimens
was withdrawn by syringe. After recording the volume
collected, the stomach content was diluted into 50 ml
with filtered seawater and re-preserved in respective
fixatives. The condition of phytoplankton cells was
observed, phytoplankton were identified until genus
level, and the cell abundance was counted using a
Sedgwick Rafter chamber at 400x magnification
according to Hakansson (2002), Hartley (1996),
Tomas (1995), Kramer and Lange-Bertalot (1986),
and Hendey (1964).
1.3 Statistical Analysis
Statistical analyses were performed using the SPSS
Windows Statistical Package (version 21). Tests were
judged to be significant at p< 0.05 level. All variables
were tested for normality and homogeneity of
variances. Data which satisfy the assumptions of
normality and homogeneity were subjected to
parametric tests, one-way ANOVA.
2 Results
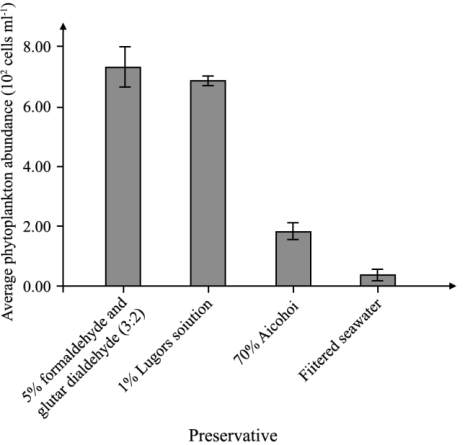
The highest phytoplankton abundance was recorded in
the gut content of mussels preserved in mixture of
formaldehyde and glutardialdehyde (7.3 x10
2
cells
ml
-1
), followed by Lugol’s solution (6.9 x10
2
cells
ml
-1
), alcohol (1.8 x10
2
cells ml
-1
) and lowest in
filtered seawater (0.35 x10
2
cells ml
-1
) (Figure 1).
There was no significant difference (P>0.05) between
the phytoplankton abundance recorded in gut of
mussels preserved with Lugol’s solution and the
mixture of formaldehyde and glutardialdehyde.
However, these values were significantly higher than
the average cell abundance recorded in gut content
preserved with 70% alcohol and filtered seawater
(P<0.05).
In general, the genus
Cascinodiscus
sp. was
dominated the phytoplankton community in the gut
content that preserved in all preservatives. Similar
Figure 1 Phytoplankton abundance in the gut of mussels
preserved in four difference preservatives
relative abundance of phytoplankton genus was
observed in the gut content of mussels that preserved
in formaldehyde- glutardialdehyde mixture and in
Lugol’s solution (Table 1). However, the composition
of
Bacteriastrum
sp.,
Chaetoceros
sp. and
Nitzschia
sp.
was significantly low in the gut preserved with 70%
alcohol and was negligible in the gut preserved with
cold filtered seawater.
3 Discussion
As expected, the gut content of specimens preserved
in cold filtered seawater was almost completely
degraded and very limited phytoplankton cells can be
identified. It is not surprising because phytoplankton
has been reported to have high lysis rate (Agusti et al.,
1998). The isotonic and low temperature (4 °C) of
cold filtered seawater does not effectively prevent the
microbial degradation of the organic matter in
phytoplankton. The small species of phytoplankton
with larger surface area per unit volume particularly
Bacteriastrum
sp.,
Chaetoceros
sp., and
Nitzschia
sp.
were degraded rapidly and resulting in alteration of
the overall relative abundance of phytoplankton in the
gut sample. The highest relative abundance of
Cascinodiscus
sp. in all gut samples could be due to
selective ingestion of green mussels (Sivalingam,
1977) or the rigid cell walls of the
Cascinodiscus
sp.
that resistant to enzymatic digestion and physical
breakdown (Romberger and Epifanio, 1981).

