Basic HTML Version

International Journal of Marine Science 2014, Vol.4, No.50, 1-22
http://ijms.biopublisher.ca
2
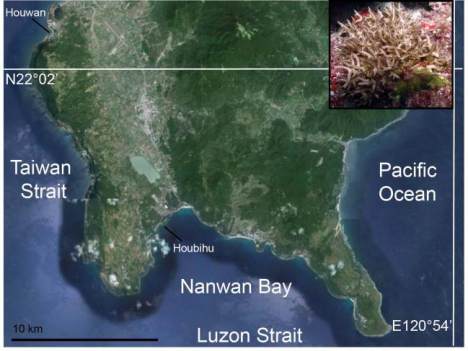
Figure 1 Map of Southern Taiwan’s Hengchun Peninsula,
including the locations of the two study sites (Houwan and
Houbihu), alongside an image of the target organism
Seriatopora hystrix,
which is common at both sites. For a
detailed treatise on the temperature environment of the two
sites, readers are pointed towards Mayfield et al. (2012a).
Briefly, the average monthly temperature ranges of Houbihu
and Houwan from 2009 to 2010 were 6.33±2.03 and
3.19±0.61
℃
, respectively, and this represents a significant
difference (student’s
t
-test,
t
=5.13,
p
<0.01). The size of the
S.
hystrix
colony in the inset is approximately 15 cm in diameter.
to be a stress-inducing temperature given that it is
~1
℃
greater than the average summer temperature of
Houbihu (Figure 1), the upwelling site (UWS) within
Nanwan Bay (Taiwan’s southernmost embayment)
from which the experimental corals were collected;
briefly, previous studies (e.g., Coles and Brown, 2003;
Hoegh-Guldberg and Smith, 1989) have found that
extended exposure to temperatures >1
℃
above the
mean summer high can result in bleaching in many
reef coral species.
Given these results, Mayfield et al. (2011) hypothesized
that
S. hystrix
residing in the UWS may have special
adaptations for life in thermodynamically variable
environments, as the temperature at the UWS can
change by up to 9
℃
in a single day during spring-tide
upwelling events that occur throughout the year (but
mainly in the northern summer; Mayfield et al.,
2012a). Mayfield et al. (2012a, 2013c) attempted to
test this hypothesis with a laboratory-based reciprocal
transplant in which corals from this UWS were
exposed to either a variable (23-29
℃
over a 5-h
period) or stable (26
℃
) temperature treatment while
conspecifics from a non-upwelling site (i.e., NUWS)
characterized by relatively stable (over diel and annual
timescales) temperatures, Houwan (Figure 1), were
exposed to the same two temperature regimes for
seven days. It was hypothesized that corals
transplanted to a “foreign” temperature regime would
be physiologically compromised in this variable
temperature experiment (VTE), though this hypothesis
was confirmed only upon assessment of coral growth
(Mayfield et al., 2012a); other parameters appear to
have been more significantly influenced by the
temperature regime alone.
To gain more insight into the genetic basis underlying
the phenotypic plasticity of these Taiwanese
S. hystrix
populations, expression of 14 gene mRNAs whose
respective proteins and cellular pathways have been
hypothesized to be disrupted upon exposure to
elevated temperature was measured in samples of the
ETE and VTE (Table 1); these included mRNAs
encoding proteins involved in photosynthesis,
metabolism, osmoregulation, the cytoskeleton, and the
stress response (see the Materials and Methods for
detailed rationale for choosing the respective genes.).
Gene expression was hypothesized to remain similar
over time and between treatments in samples from the
UWS exposed to 30
℃
for two days in the ETE given
the lack of
hsp70
modulation documented at these
temperatures by Mayfield et al. (2011). In contrast, it
was hypothesized that the majority of the genes would
be expressed at different levels between the stable and
variable temperature treatments in the VTE in corals
from the UWS only, given that exposure to a familiar
temperature change can drive significant changes in
mRNA expression in other marine organisms (e.g.,
Gracey et al., 2008). It was further hypothesized that
corals from the UWS would demonstrate higher levels
of target gene expression than conspecifics from the
NUWS. Briefly, corals inhabiting variable temperature
environments could be expected to express higher
mRNA levels; the reasoning behind this is due to the
need for rapid increases in protein translation at times
at which temperature increases have incapacitated the
standing pool of intracellular proteins in response to
elevated temperature-induced protein denaturation
(Hazel and Prosser, 1974). This is a strategy employed
by not only intertidal limpets (Dong et al., 2008), but
also other corals (Barshis et al., 2013).

