Basic HTML Version

International Journal of Marine Science 2013, Vol.3, No.30, 238-243
http://ijms.sophiapublisher.com
242
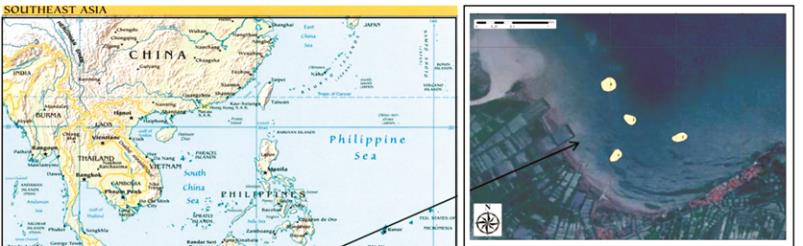
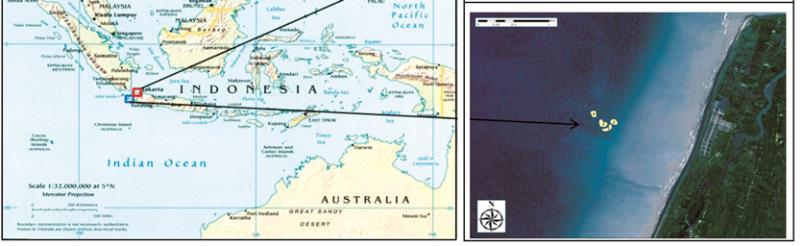
Figure 4 Sampling stations of sediments and bivalve mollusc (
Anadara indica
) at Tanjung Pasir (above-right) and Panimbang
(below-right)
3) Oxidisable-organic (Fraction 3): The residue from
(2) was first oxidized with 15 mL hydrogen peroxide
(30%) in a water bath at 80
℃
. After cooling, the metal
released from the organic complexes was continuously
shaken for 3 h with 50 mL of 1.0 M ammonium
acetate (NH
4
CH
3
COO) acidified to pH 2.0 with HCl,
at room temperature.
4). Resistant (Fraction 4): The residue from (3) was
digested in a 10 mL combination (ratio of 3:1 of
concentrated nitric acid and perchloric acid).
4.4 Heavy metals analysis in
Anadara indica
About 1 g of the soft tissues of
A. indica
were
thoroughly washed with Milli-Q water, and extracted
for further processing. Soft tissue was macerated into
1-2 cm clumps, dried at 70
℃
~80
℃
, grinded and
stored until chemical analysis. Copper (Cu), Lead (Pb),
and Zinc (Zn) were analyzed by digesting the
homogenized samples in a mixture of nitric and
perchloric acids. After being centrifuged the supernatant
was filtered through syringe filter paper (45 µm).
After filtration, heavy metals were determined for Cu, Pb
and Zn using an air-acetylene flame atomic absorption
spectrophotometer (AAS) Shimadzu Model 6300 series.
4.5 Statistical Analyze
Statistical analyses were performed using Statistical
Package for Social Science (SPSS) version 20. The
Spearman’s rank correlation coefficient was applied in
order to determine the strength and significance of the
relationships between the concentrations of Cu, Pb,
and Zn in
A. indica
with the geochemical fractions of
respective metals in the sediment.
References
Arifin Z. 2005. Heavy Metal in Pollution in Sediments of Coastal Waters of
Indonesia. Jakarta: Research Center of Oceanography LIPI
Belcheva N.N., Zakhartsev M., Silina A.V., Slinko E.N., and Chelomin V.P.,
2006, Relationship between shell weight and cadmium content in
whole digestive gland of the Japanese scallop
Patinopecten yessoensis
(Jay). Mar. Environ. Res. 61: 396-409
http://dx.doi.org/10.1016/j.marenvres.2005.12.001
Bryan G.W. and Langston W.J., 1992, Bioavailability, accumulation and
effects of heavy metals in sediments with special reference to United
Kingdom estuaries: a review. Environ. Pollut., 76: 89-131
http://dx.doi.org/10.1016/0269-7491(92)90099-V
Bruland K.W., Franks R.P., Knauer G.A., and Martin J.H., 1979, Sampling
and analytical methods for the determination of copper, cadmium, zinc,
and nichel at the nanogram level in sea water. Anal. Chem. Acta., 10:
223-245
Bubb J.M., Rudd T., and Lester J.N., 1991, Distribution of heavy metals in
the River Yare and its association broads. III. Copper and Cadmium. Sci.
Tot. Environ., 102:169-188
http://dx.doi.org/10.1016/0048-9697(91)90313-4
http://dx.doi.org/10.1016/0048-9697(91)90314-5
http://dx.doi.org/10.1016/0048-9697(91)90312-3
Cairns J., 1984. Factors moderating toxicity in surface waters; In Wlison J.
(ed.) The Fate of Toxics in Surface and Ground Waters. Acad. Nat. Sci
Philadelphia, pp. 49-64
Davies-Colley R.J., Nelson P.O., and Williamson K.H., 1984, Copper and
cadmium uptake by estuarine sedimentary phase. Envir. Contam.
Toxicol., 30:491-499
Erickson R.J., Benoit D.A., Mattson V.R., Nelson H.P., and Leonhard E.N.,
1996, The effects of water chemistry on the toxicity of copper to
fathead minnows. Environ. Sci. Technol., 15: 181-193

