Basic HTML Version

International Journal of Marine Science 2013, Vol.3, No.27, 212-218
http://ijms.sophiapublisher.com
215
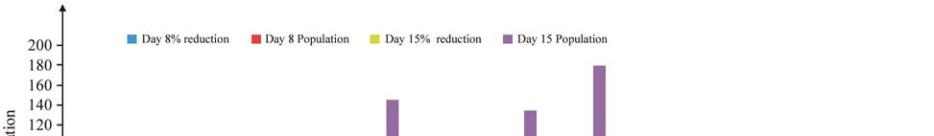
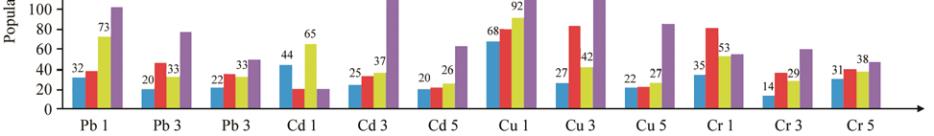
Figure 2 The percentage of Pb, Cd, Cu, and Cr reduction on the culture media of
Porphyridium cruentum
(S.F. Gray) Nägeli
in day 8
and 15 treatment concentration 1, 3, 5 mg/L
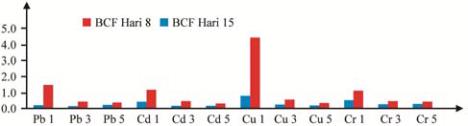
Heavy metals toxicity can be study by BCF approach.
The highest BCF occurred for heavy metals treatment
on the concentrations of 1 mg/L (Figure 3). However,
the length of treatment influenced BCF value.
P.
cruentum
shown the higher toleration on Cu than Pb,
Cd, and Cr. BCF of
P. cruentum
in day of 8 from high
was in order of Cu > Cr > Cd > Pb, respectively;
however, in day 15 was Cu > Pb > Cd > Cr.
Figure 3 BCF (ppm) of Pb, Cd, Cr, Cu on the concentration 1, 3,
and 5 mg/L
Heavy metals bioconcentration on the
P. cruentum
able to figure out the environmental impact of heavy
metals. Based on the trend in Figure 3, it seems that
Pb required longer time to accumulate, whereas Cu
was more faster.
BCF had calculated as a homeostatically ratio of
heavy metal concentration on the
P. cruentum
with
heavy metal concentration of media. Microalgae had a
protection mechanism against heavy metals by
development of heavy metals complex with cellular
protein without change its activity (Wang and Chen,
2009; Girard, 2010). On a high concentration, heavy
metals had reduced the population or cell growth
because
P. cruentum
can not counterbalance the heavy
metals toxicity.
The mechanism of heavy metal entering to the cell
was affected by the concentration difference, the
negative charged of the surface cell wall and the
positive charged metal ion on the microalgal medium.
It was shown from this study that
P. cruentum
demonstrated successfully in the sorption and removal
of heavy metals ion from the water, the highest
affinity towards Cu which in the day of 8 and/or 15. A
reduction of Cu concentration was higher following
the time exposure, which accumulate in the cell wall.
This was related to the Cu release rate that relatively
lower than the Cu absorption. The heavy metals
absorption occurred in 2 ways i.e. heavy metal ionic
change with cell wall caption, or development
covalent bound between heavy metals with active
ionic of cell wall.
P. cruentum
cell wall consists of
organic protein, polysaccharide, alginate acid and
urinate acid which were able to bind with heavy
metals (Wang and Chen, 2009).
Heavy metal accumulation will increase H
+
ion
concentration. Therefore, an increase of pH media will
increase H
+
ion production, which in turn will increase
heavy metal absorption by
Porphyridium
. So, heavy
metals bioremediation by
P. cruentum
will be
optimum on the alkaline pH (7-8) condition.
Many researches had been done on the effect of heavy
metals on the microalgae. A high concentration of Pb
and Cd had decreased
Cladophora fracta
growth, due
to induction of peroxides enzyme activity that had an
important role on the indoneacetic acid (IAA)
degradation. IAA was a hormone that stimulates the
growth and vision of microalgae (Lamai, et al., 2005).
Chlamydomonas reinhardtii
,
Chlorella salina
,
Chlorella
sorokiniana
,
Chlorella vulgaris
,
Chlorella miniata
,
Chlorococcum
sp.,
Cyclotella cryptica
,
Lyngbya
taylorii
,
Phaeodactylum tricornutum
,
Porphyridium

