Basic HTML Version


Int'l Journal of Marine Science 2012, Vol.2, No.9, 62
-
69
http://ijms.sophiapublisher.com
68
(Crawford et al., 1986). After zooxanthellae offering,
artificial lighting must be turned on in indoor systems.
Photoperiod should simulate tropical reef conditions
and light distribution must be homogenous across the
raceways. Considering veliger larvae are positively
phototactic, patched lighting may result in aggregation
and overcrowding in some areas. Daily sampling for
the determination of zooxanthellae acquisition by
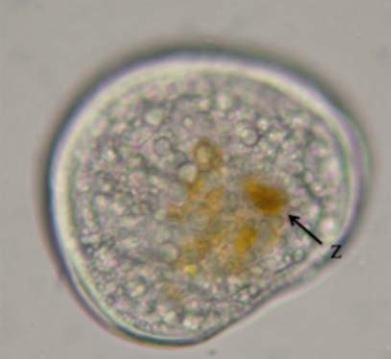
veliger larvae is recommended (Figure 3).
Figure 3 Veliger larva of
Tridacna crocea
with defined
calcareous shells at ca. 100 h post-fertilization. Digestive tract
is partially full containing several zooxanthellae cells (Z).
4.3 Pediveliger, metamorphosis and grow-out
Pediveliger larvae require as much attention as that
provided for the veliger stage. Feeding and
zooxanthellae additions once every two days greatly
improve survival (Crawford et al., 1986; Mies et al.,
2012). At approximately 14 days pf the
metamorphosis is complete and a thin mantle will be
extended beyond the shells and visible. At this point
the substrate can be removed and transferred to the
grow-out tank for seed production. Strong lighting and
intense water movement aid in the growth of juvenile
clams, as well as slightly elevated nutrient
concentrations (Fitt et al., 1993). It has been shown
that a concentration of approximately of 4.0×10
-4
g L
-1
of NH
4
NO
3
stimulates zooxanthellae growth and
significantly increases shell length (Heslinga et al.,
1990; Grice and Bell, 1999).
In aquaculture establishments near the shore, juveniles
at 2 cm of shell length can be transferred to open
ocean grow-out cages (Calumpong, 1992). It has been
determined that giant clams tend to grow much faster
in natural conditions (Munro et al., 1993). Large cages
made with PVC or plastic can be placed in clear,
shallow and quiet tropical areas and stock individuals
at 1,000 individuals m
-2
(Heslinga et al., 1984). Covering
the cages with 25-mm mesh is recommended to avoid
predation by fish and benthic invertebrates
(Calumpong, 1992). The cages are no longer
necessary once clams reach 20 cm in shell length
considering that the risk of predation is significantly
reduced at this point.
Authors’ Contributions
M Mies and PYG Sumida both wrote and reviewed the presented article.
Acknowledgements
We would like to sincerely thank all of our many colleagues that have
participated and contributed with our giant clam experiments, including the
staff of both the Oceanographic Institute of the University of São Paulo
(IO-USP) and of the Foundation of Aquatic Studies and Research
(FUNDESPA).
References
Beckvar N., 1981, Cultivation, spawning, and growth of the giant clams
Tridacna gigas
,
T. derasa
and
T. squamosa
in Palau, Caroline Islands,
Aquaculture, 24: 21-30
http://dx.doi.org/10.1016/0044-8486(81)90040-5
Braley R.D., 1984, Reproduction in the giant clams
Tridacna gigas
and
Tridacna derasa
in situ on the north-central Great Barrier Reef,
Australia, and Papua New Guinea, Coral Reefs, 3: 221-227
http://dx.doi.org/10.1007/BF00288258
Braley R.D., 1985, Serotonin-induced spawning in giant clams (Bivalvia:
Tridacnidae), Aquaculture, 47: 321-325
http://dx.doi.org/10.1016/0044-8486(85)90217-0
Braley R.D., 1988, Recruitment of the giant clams
Tridacna gigas
and
T.
derasa
at four sites on the Great Barrier Reef, In: Copland J.W., and
Lucas J., (eds.), Giant Clams in Asia and the Pacific, ACIAR
Proceedings nº 9, Australian Centre for International Agricultural
Research, Canberra, pp.73-77
Braley R.D., ed., 1992, The Giant Clam: A Hatchery and Nursery Culture
Manual, 1
st
edition, Australian Centre for International Agricultural
Research, Canberra, pp.9-13
Calumpong, H.P., ed., 1992, The Giant Clam: An Ocean Culture Manual, 1
st
edition, Australian Centre for International Agricultural Research,
Canberra, pp.7-13
Crawford C.M., Nash W.J., and Lucas J.S., 1986, Spawning induction, and
larval and juvenile rearing of the Giant Clam,
Tridacna gigas
,
Aquaculture, 58: 281-295
http://dx.doi.org/10.1016/0044-8486(86)90094-3
Ellis S., ed., 1998, Spawning and early larval rearing of giant clams
(Bivalvia: Tridacnidae), 1
st
edition, Center for Tropical and Subtropical
Aquaculture publication nº 130, Waimanalo, pp.1-55
Fitt W.K., 1993, Nutrition of giant clams, In: Biology and Mariculture of
Giant Clams, W.K. Fitt (ed.), ACIAR Proceedings nº 47, Australian
Centre for International Agricultural Research, Canberra, pp.31-40

