Basic HTML Version


Int. J. of Marine Science 2012, Vol.2, No.6, 43
-
50
http://ijms.sophiapublisher.com
46
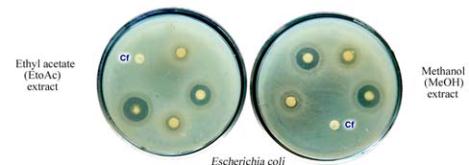
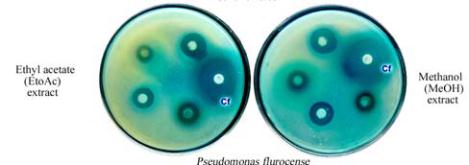
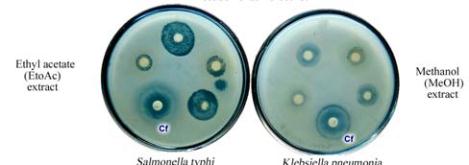
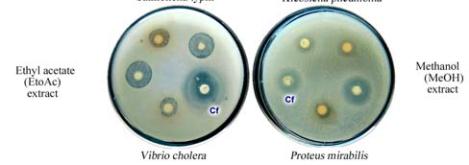
Figure 2 Antimicrobial screening and antibacterial activity of
well assay method
Antifungal activity was also observed in the extracts
of the sponges.
C. albicans
was sensitive to all the
extracts tested and
S. cerevisiae
was resistant to both
ethyl acetate and methanol extracts of sponges. The
presence of chemical constituents like steroids, Tri
terpenoids, Reducing sugar, Alkaloids, phenolic
compound, Saponin, Xantho protein, Tannin,
Flavanoids and Aromatic acid, were tested in the
selected four species of sponges (Table 3).
Steroid was present in all the four species except in
A.
globostellata
(TCN-8), triterpenoid was present only
in
reducing sugar was absent in
A. globostellata
(TCN-8) alkaloids, saponin and flavanoids were
present in the species
A. globostellata
(TCN-8),
phenolic compound, xantho protein, aromatic acid
were absent in all the species was present in
S.
inconstans
var.
moeandrina
(TCN-10)
S. inconstans
var.
globosa
(RSM-13).
This is not surprising as the sponge belonging to this
genus and collected from different regions is reported
to possess wide variety of compounds with different
biological activities. Thus,
Haliclona
sp. from
Indonesia yielded a triterpene ketide, Halicotriol B
with weak antimicrobial activity against
S. aureus
and
Bacillus subtilis
(Crews and Harrison, 2000). The
antifungal papuamine has been reported by Baker et al
(1988) from a
Haliclona
sp. Fahy et al (1988) report
a major antimicrobial alkaloid haliclonadiamine
together with antifungal papuamine from
Haliclona
sp.
of Palau. Antifungal aminoalcohols have been
identified from a new species of
Haliclona
from
Queensland (Clark et al., 2001). Charan et al (1996)
report antimicrobial Haliclonacyclamines. It is
therefore expected that the activity found by us in the
extract of
H. cribricutis
could have, at least partially,
been contributed by any one of the above compounds
isolated from this genus. Organisms belonging to the
same genus are bound to have common chemical
constituents. Parameswarn et al (1992) report
significant anti-viral and antibacterial activities in
petroleum ether and ethyl acetate fractions of
H.
cribricutis
and the activity observed against
K.
pneumoniae
and
Vibrio
parahaemolyticus
was
attributed to o-demethyl renierones.
Ircinia
sp.
exhibited mild antibacterial activity only against
S.
aureus
but all the fungal strains tested were insensitive
to it.
A number of cytotoxic compounds are reported from
this genus. These include 73
-
deoxychondropsin-A
from an Australian
Ircinia ramosa.
Chondropsin-C
was found in a Philippine
Ircinia
species (Rashid et al.,
2001). Moderately cytotoxic cumulated ketene
irciniketene has been reported from
Ircinia selaginea
collected from Guangxi Province, China (Yan et al.,
2001). Cytotoxic kohamaic acids A and B are known
to be constituents of Ircinia species from Okinawa
(Kokubo et al., 2001). Three tricarbocyclic
sesterterpenoids of the cheilanthane class isolated
from a Queensland Ircinia species were found to be
inhibitors of MSK-1 and MAPKA-2 protein kinases
(Buchanan et al., 2001). Though cytotoxic compounds
are reported from this genus, there are no reports of
any antimicrobial activity in the extracts.

