Basic HTML Version


Bt Research 2012, Vol.3, No.1, 1
-
2
http://bt.sophiapublisher.com
2
a mitigating factor for
Bt
to grow in the absence of
insects. Such soil-plant life cycle might not exclude
insects, since multiplication in the bodies as well as in
the frass of surviving insects has been reported (Bizzarri
and Bishop, 2008; Prabhakar and Bishop, 2009), and this
would certainly be a supplementary source of spores to
re-inoculate soil and then plants through young leaflets
colonization. Additionally, since colonized plants are at
least partially protected against phytofagous insects
(Prabhakar and Bishop, 2009), a plant symbiont-like
role for
Bt
can also be considered.
The analysis of these and similar reports, taken together,
lead us to define a new life cycle for
Bt
that combines
the insect-based and insect-independent cycles mentioned
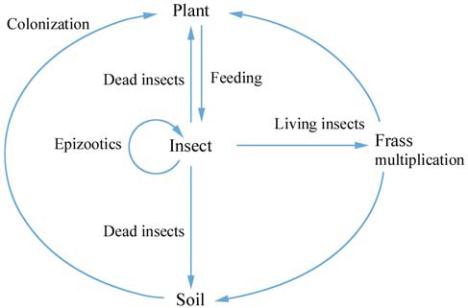
above. The proposed cycle, shown in Figure 1,
integrates plants, frass and soil as sub-cycles of a main
insect-centered cycle. But the key question still
remains unanswered: is
Bt
a real insect pathogen? We
think that, beyond the reported epizootics outbreaks, a
major argument proving that
Bt
is indeed an insect
pathogen is the elegant specificity of the arsenal of
Bt
insecticidal toxins active on Lepidoptera, Coleoptera,
Diptera and also other invertebrates such as nematodes,
which is an obvious adaptive character. But the
well-documented insect-free plant-based cycles, horizontal
gene transfer phenomena such as
Bt
Cyt genes
identified in the aphid pathogen and phytopathogenic
bacterium
Dickeya dadantii
(Grenier et al., 2006), or
the ease with which
Bt
can be grown in the lab strongly
reveal that
Bt
is, additionally to an insect pathogen, also
an opportunistic non-pathogen microorganism able to
persist and/or multiply on a range of substrates
including feces or plant surfaces from which it might
re-enter its basic insect-centered life cycle.
Multiplication can be carried out in insects, but also in
frass or plant surfaces. Soil may play a central role as a
reservoir of spores. The presence of
B. thuringiensis
on
plants can originate from colonization of leaflets from
soil, spreading of frass on the leaves from living insects
or from decomposition of spore-rich insect bodies,
particularly after epizootics outbreaks. Notice that
aquatic strains such as
B. thuringiensis
var.
israelensis
may display a variation of this with a simpler insect (i.e.
mosquito larvae)-freshwater cycle.
Figure 1 Proposed
life cycle for
Bacillus thuringiensis
Authors' contributions
GM and MP had contributed equally to the work reviewing
bibliography and writing and revising the article.
Acknowledgements
We thank two anonymous reviewers for their help in preparation
of manuscript.
References
Bizzarri M.F. and Bishop A.H., 2008, The ecology of
Bacillus thuringiensis
on
the Phylloplane: colonization from soil, plasmid transfer, and
interaction with larvae of
Pieris brassicae
, Microb. Ecol., 56(1):
133-139 http://dx.doi.org/10.1007/s00248-007-9331-1 PMid:17973155
Broderick N.A., Raffa K.F., and Handelsman J., 2006, Midgut bacteria
required for
Bacillus thuringiensis
insecticidal activity, Proc. Natl.
Acad. Sci., USA, 103(41): 15196-15199 http://dx.doi.org/10.1073/pnas.
0604865103 PMid:17005725 PMCid:1622799
De Barjac H., 1978, A new variety of
Bacillus thuringiensis
very toxic to
mosquitoes: B. thuringiensis var. israelensis serotype 14, C.R. Acad.
Sci. Hebd. Seances Acad. Sci. D., 286: 797-800 PMid:417869
Grenier A.M., Duport G., Pagès S., Condemine G., and RahbéY., 2006, The
phytopathogen
Dickeya dadantii
(
Erwinia chrysanthemi<

